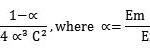2, 4 Dihydroxy-5-Bromo[2’-Methyl] Propiophenone Oxime as an Analytical Reagent: Studies on Cu(II) Chelate
Nitinkumar B. Patel and Nirav Hiteshkumar Parekh
Shree Jayendrapuri Arts and Science College, Bharuch - 392 002, India.
Article Received on :
Article Accepted on :
Article Published : 21 Oct 2016
Cu(II) was determined spectrophotometrically after co-precipitation with 2, 4 Dihydroxy-5-bromo[2’-methyl] propiophenone oxime (DHBMPO) at room temperature. Obeyance of Beer’s law was found and from that Molar absorptivity and Sandell’s sensitivity were calculated. Composition of chelate was determined using Job’s method of continuous variation and Yoe and Jones mole ratio method. The stability constant and Gibb’s free energy change were also determined spectrophotometrically. From TGA, the energy of activation was also calculated using Broido method for the first and second step decomposition. The reagent has been satisfactorily applied for the determination of copper in drain micro etch solution.
KEYWORDS:Propiophenone oxime; DHBMPO; Chelate
Download this article as:| Copy the following to cite this article: Patel N. B, Parekh N. H. 2, 4 Dihydroxy-5-Bromo[2’-Methyl] Propiophenone Oxime as an Analytical Reagent: Studies on Cu(II) Chelate. Orient J Chem 2012;28(4). |
| Copy the following to cite this URL: Patel N. B, Parekh N. H. 2, 4 Dihydroxy-5-Bromo[2’-Methyl] Propiophenone Oxime as an Analytical Reagent: Studies on Cu(II) Chelate. Available from: http://www.orientjchem.org/?p=22777 |
Introduction
Several organic compounds with phenolic protogenic group and a suitably placed electron donating atom like nitrogen, oxygen or sulphur are found to interact with metal ions giving precipitation or coloration due to complex formation. Many organic reagents like o-hydroxy oximes1-7, oximes8-9, thiosemicarbazones10-14, chalcone oxime15, anilides16, various heterocyclic compounds have been used for gravimetric and spectrophotometric determination of metal ions. Oximes are selective and sensitive reagents for this purpose and hence here we had introduced a novel reagent (DHBMPO) for the determination of Cu(II).
Experimental
DHBMPO was synthesized using the method of H. Nogami17 by the condensation of resorcinol with isobutyric acid in presence of anhy. ZnCl2 giving 2, 4 dihydroxy [2’-methyl] propiophenone. It was than brominated with Bromine in acetic acid giving 2, 4 dihydroxy-5-bromo[2’-methyl] propiophenone. Bromo derivative was then converted to oxime using hydroxyl amine hydrochloride and sodium acetate. The oxime was re-crystallized from ethanol bearing M.P. 162.1º C.
Cu(II)-metal solution: A stock solution of copper(II) (0.05M) was prepared by dissolving an accurately weighed amount of Copper sulphate penta hydrate in deionized water.
Solutions of other diverse ions were prepared by dissolving their salts (A.R.) in deionized water.
Characterization Of reagent
Elemental analysis
Elemental analysis of the reagent was done using Elementar Vario EL III analyzer. The percentages are in agreement with its molecular formula. The results are represented in Table:1
Table 1 : Elemental analysis of Reagent
|
Reagent |
Percentage found (Calculated) |
||
|
DHBMPO |
Carbon |
Hydrogen |
Nitrogen |
|
43.55% (43.79%) |
4.367% (4.379%) |
5.020% (5.109%) |
|
UV-Visible spectral studies
The UV-Visible spectrum of reagent in ethanol was recorded on “Perkin-Elmer Lambada-35 UV-Visible spectrophotometer”. Wavelength of maximum absorption is 226 nm.
FT-IR Spectral studies
FTIR spectrum of the reagent was recorded on “Perkin Elmer-Spectrum RX-I Spectrophotometer” in KBr pellet. The bands which are observed are given in Table:2
Table 2: IR Spectra
|
Reagent |
ν(O-H) Phenolic |
ν(O-H) Oximino |
ν(C-H) aliphatic |
ν(C=C) aromatic |
ν(C=N)
|
ν(N-O)
|
|
DHBMPO |
3484 cm-1 |
3389 cm-1 |
2965 cm-1 |
1599 cm-1 |
1633 |
927 |
1H-NMR Spectral studies
The NMR spectrum of the reagent was taken in DMSO. The NMR spectrum was recorded on Brucker Avance-II400 NMR spectrophotometer using TMS as reference. Assignment of signals to different protons is given in Table:3
Table 3: NMR Spectra
|
Reagent |
Alkyl group |
Methine Proton |
Phenolic (-OH) |
Oximino (-OH) |
Aromatic proton |
|
DHBMPO |
1.3680-1.3855 ppm (singlet) |
3.3836-3.4531 ppm (multiplet) |
12.9897 ppm |
11.2118 ppm |
7.5682 ppm |
Results And Discussion
Gravimetric determination Of Cu(II)
A 0.05 M solution of the reagent in 50% aqueous ethanol was used. Copper sulphate solution (0.05 M, 10ml) was taken in a clean beaker. A (0.05 M, 22ml) ligand solution was added and diluted with double distilled water. Different sets were prepared and studied within the pH range of 3.0-6.0. pH of the solution was adjusted using Sodium acetate-acetic acid buffer. A buff precipitate were obtained were digested on water-bath for 60 minutes at 60°C. The precipitate were filtered through a previously weighed sintered glass crucible (G4) and washed with warm water followed by 50% aqueous ethanol to remove excess of the reagent which might have precipitated on dilution. The chelate was dried to constant weight at 110-115°C in hot air oven, cooled and weighed. The experiment was repeated at different pH of solution. The results obtained are given in Table:4
Table 4: Gravimetric determination Of Cu(II)-DHBMPO
|
pH |
Cu(II) complex in gm |
Cu(II) found in mg |
Error |
|
|
in mg |
% |
|||
|
3.0 |
0.3016 |
31.4388 |
-0.3312 |
-1.04 |
|
3.0 |
0.3013 |
31.4075 |
-0.3625 |
-1.14 |
|
3.5 |
0.3018 |
31.4596 |
-0.3104 |
-0.98 |
|
3.5 |
0.3020 |
31.4805 |
-0.2895 |
-0.91 |
|
4.0 |
0.3041 |
31.6994 |
-0.0706 |
-0.22 |
|
4.0 |
0.3043 |
31.7202 |
-0.0498 |
-0.16 |
|
4.5 |
0.3064 |
31.9391 |
+0.1691 |
+0.53 |
|
4.5 |
0.3068 |
31.9808 |
+0.2108 |
+0.66 |
|
5.0 |
0.3073 |
32.0330 |
+0.2630 |
+0.83 |
|
5.0 |
0.3077 |
32.0746 |
+0.3046 |
+0.96 |
|
5.5 |
0.3085 |
32.1580 |
+0.3880 |
+1.22 |
|
5.5 |
0.3086 |
32.1685 |
+0.3985 |
+1.25 |
|
6.0 |
0.3094 |
32.2519 |
+0.4819 |
+1.52 |
|
6.0 |
0.3093 |
32.2414 |
+0.4714 |
+1.48 |
Effect Of diverse ion
To study the effect of foreign ions on gravimetric determination of Cu(II), 8 to 10 mg of various cations were added to a solution containing 31.77 mg of Cu(II) at pH 4.0 and gravimetric estimation were carried out. It was observed that Mg(II), Sr(II), Mn(II), Cd(II), Ca(II), Ni(II), Zn(II), Ba(II) do not interfere at this pH but Fe(III) and Pd(II) interfered seriously. Interference of Fe(III) can be removed by masking it with H3PO4. Many common anions like nitrate, nitrite, sulphate, chloride, bromide and iodide were not found to interfere.
Spectrophotometric study Of Cu(II)-DHBMPO
In absorption spectra of Cu(II) complex in chloroform a shoulder band is obtained at 410nm and hence all spectrophotometric measurements were done at this wavelength. Different aliquots of Cu(II) solution were taken and buffer solution was added to maintain the pH 4.0. The excess reagent was added to get complete precipitation of complex. It was extracted in three 5.0ml portion of chloroform and final volume of this solution was adjusted to 25ml with chloroform. The absorbance was measured at 410nm and plotted against the concentration of Cu(II). It was found that Beer’s law was obeyed upto 25.42 ppm of Cu(II). Molar absorptivity and Sandell’s sensitivity were calculated form graph and it was found to be 2.7 x 103 lit mol-1 cm-1 & 0.0234 µg/cm2 respectively. Job’s method of continuous variation18 and Yoe and Jones mole ratio method19 were used to determine the stoichiometry of the complex. It was found to be 1:2[M:L]. This is in agreement with stoichiometry determined from gravimetric analysis. The stability constant was calculated using the formula,
where, α= degree of dissociation
Em=maximum absorbance found from graph.
Es =absorbance at the stoichiometric molar
ratio of the metal to reagent in complex.
C = concentration of complex
The average stability constant from the above two methods was found to be 1.85×1010 and ∆G° for complex formation reaction at 27°C was found to be -14.0934 k cal/mol.
Thermogravimetric analysis
From TG analysis of the Cu(II) chelate, it was found that there is no weight loss upto 191°C indicating that the chelate can be dried safely without decomposition at 110°C. Loss in weight from 191º-860ºC is due to removal of organic ligand molecules. Observed loss and weight of metal residue agrees well with the formula of the chelate in which M:L ratio is 1:2. Activation energy Ea was calculated using Broido method20 and found to be 27.897 k cal/mol for first step and 2.998 k cal/mol for second step decomposition.
IR Spectra
Interpretation of IR-spectra of the chelate shows weak band around 3485 cm-1 in comparison of ligand molecule. The weak band due to ν-OH of oximino group is found at 3389 cm-1 in ligand, is observed at nearly the same position in the complex. νC=N stretching band observed at 1633 cm-1 in ligand is shifted to 1617 cm-1 in chelate, this indicates nitrogen is coordinately bonded with metal ion and covalently bonded with oxygen atom. It is also supported by the downward shift of νN-O group i.e. from 927 to 880 cm-1.
Spectrophotometric determination Of Cu(II)
Determination of Cu(II) was done in drain micro etch solution. From conventional titrimetric method % of Cu(II) was found to be 2.351% in original sample solution. 10ml of stock solution was diluted to 100ml.10ml of this solution was further diluted to 100ml. From that 25ml of solution was finally diluted to 50ml. A 2.5ml and 3.5ml aliquots of this diluted sample was taken in two different beakers containing 4.0ml (0.01M) of reagent solution each. Small amount of double distilled water was added and pH of the solution was adjusted to 4.0 with sodium acetate and acetic acid buffer. Complex was extracted in CHCl3 and final volume was made 25ml. The absorbance was measured and ppm of Cu(II) was calculated by using Beer’s law plot. Results obtained are given in Table:5 and Fig.1.
Table 5: Determination Of Cu(II) in drain micro etch solution
|
Cu(II) taken in ml |
Absorbance
|
ppm found |
ppm taken |
% Cu(II) found |
% Error
|
|
2.5 |
0.492 |
11.65 |
11.755 |
2.3300 |
–0.89 |
|
3.5 |
0.696 |
16.35 |
16.457 |
2.3357 |
–0.65 |
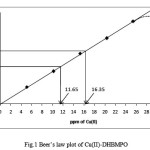 |
Figure :1 Beer’s law plot of Cu(II)-DHBMPO |
Acknowledgement
The authors are thankful to The Principal, Shree Jayendrapuri Arts & Science college, Bharuch and The Head, Department of chemistry, Shree Jayendrapuri Arts & Science college, Bharuch for providing the facilities to carry out the research work.
References
- Desai K.K. & Naik H.B., Indian J. Chem., 25A, 297(1986).
- Patel N.B. & Desai K.K., Asian J. Chem., 15, 751(2003).
- Patel N.B. & Desai K.K., Asian J. Chem., 16, 1076(2004).
- Naidu R. Sheshadri & Naidu R. Raghava, Talanta, 25, 354(1978).
- Shingadia S.K. & Desai K.K., E.J. Chem., 4(1), 97(2006).
- Shingadia S.K. & Desai K.K., Oriental J. Chem., 22(3), 703(2006).
- Patel V. M. & Patel N. B., Acta Ciencia Indica, Vol. XXXIV C, No. 3, 427(2008).
- Ninan S., Varadarajan A., Jadhav, S.B., Kulkarni, A.J. & Malve S.P., Spectrochimica Acta Part A, 55, 825(1999).
- Wiersma Lawrance K. & Cott Peter F., Analytica Chimica Acta, 40, 291(1968).
- Desai M.J. & Desai K.K., Asian J. Chem., 11(4), 1313-1316(1999).
- Reddy K. Hussain, Prasad N.B.L. & Reddy T. Shreenivasalu., Talanata, 59, 423(2003).
- Pavon J.M. Can. & Pino F., Talanta, 20, 339(1973).
- Satheesh K.P., Ravichandran. S, Rao V.Suryanarayana, Devanna N. and Chandrasekhar K.B., International Journal of ChemTech Research, 3, 4, 1740-1746(2011).
- Lokhande R. S., Janwadkar S. P., Pitale Shirish, Kulkarni Santosh, Patil Sanjay, International Journal of ChemTech Research, 3, 4, 1765-1768(2011).
- Desai A.M. & Desai K.K., Journal of SGU, 1, 16(2003).
- Chaudhari N.K. & Das J., Analytica Chimica Acta, 57, 193(1971).
- Nogami H., Pharm. Soc., Japan, 61, 46(1945).
- Job P., Ann. Chem., 9, 113(1928).
- Yoe J. H. & Jones A. L.; Ind. Engg. Chem. Anal. Ed. 16, 111(1944).
- Broido A., J. Polym. Sci., Part A-2, 7, 1761(1969).

This work is licensed under a Creative Commons Attribution 4.0 International License.