Application of the Claisen Condensation Reaction to 3 Aromatic Esters that are found in Nature
Rishan Singh
Republic of South Africa
The aim of the Claisen condensation reaction is to produce ?-keto esters for the synthesis of other compounds. This article shows how the Claisen condensation reaction refutes its own aim by not producing β-keto esters, in the case of 3 aromatic esters (methyl butyrate, isopentyl acetate and benzyl acetate), yet still being successful.
KEYWORDS:Carbonyl compound; Enolate ion; Nucleophilic substitution reaction; Alkoxide ion; β-keto ester
Download this article as:| Copy the following to cite this article: Singh R. Application of the Claisen Condensation Reaction to 3 Aromatic Esters that are found in Nature. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Singh R. Application of the Claisen Condensation Reaction to 3 Aromatic Esters that are found in Nature. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=23966 |
Introduction
The Claisen condensation reaction of the 3 aromatic esters; methyl butyrate (apple) (figure 1), isopentyl acetate (banana) (figure 2) and benzyl acetate (jasmine) (figure 3), involves a nucleophilic substitution reaction (Bruice, 2007; Rishan Singh, personal statement). See figures below for the Claisen condensation reaction of the above mentioned aromatic compounds, respectively.
 |
Figure 1: Reaction initiation, and outcome, of methyl butyrate with alkoxide ion during Claisen condensation |
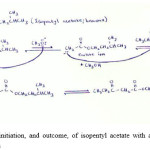 |
Figure 2: Reaction initiation, and outcome, of isopentyl acetate with alkoxide ion during Claisen condensation |
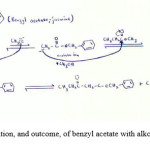 |
Figure 3: Reaction initiation, and outcome, of benzyl acetate with alkoxide ion during Claisen condensation |
A nucleophilic substitution reaction, by definition, is a chemical reaction that involves a negatively charged ion viz. a nucleophile, and an electrophile (Petrucci et al., 2002); in the case of the Claisen condensation reaction it involves, an α (alpha) carbon atom of the ester (Bruice, 2007). Furthermore, the esters involved in this reaction are esters that belong to the class one carbonyl compounds (Bruice, 2007; Rishan Singh, personal statement). When a chemical reaction has an ester possessing two hydrogens and sufficient equivalent amounts of strong base (pH > 11), Claisen condensation proceeds with a high degree of assurance. Equivalent amounts of strong base differ from the catalytic amount of the same base because catalysis involves the addition of chemical agents and/or enzymes for the reaction to reach completion with a greater reaction velocity. In the Claisen reaction, the aim is to achieve reaction completion without the need of increasing the reaction velocity, yet still maintaining a reversible reaction in the process (Rishan Singh, personal statement and writing).
When a strong base is added to all 3 carbonyl compounds, a proton is removed from the alpha (α) carbon of the compound. This causes the starting reactant (ester) to become an enolate ion. The formation of the enolate ion occurs when the lone pair of electrons of the alkoxide ion (CH3O–) attracts a hydrogen atom of the α-carbon causing the starting ester to become electron proficient, since it loses a proton. By definition, an alkoxide ion, is an ion that is formed from the joining of a methyl group to an oxygen atom bearing a negative charge (Rishan Singh, personal writing).
Once the carbonyl compound is converted to an enolate ion, the enolate ion bearing a negative charge has a high affinity for a second molecule of the ester (irrespective of the type of ester) and it attacks the carbon of the ester that is double bonded to it. In turn the double bond breaks into a single bond and the oxygen becomes negatively charged. This sequence of events is prevalent in all 3 carbonyl compounds, as can be seen in figures 1, 2 and 3. Furthermore, the base (CH3OH) is similar to the leaving group (CH3O–) in the Claisen condensation reaction. This is important in order to ensure that the Claisen reaction is balanced at equilibrium. This means that even if the base were to act as a nucleophile and attack the carbonyl group, the final product (β-keto ester) and reactant will still be the same (Rishan Singh, personal statement). However, it is important to note that the forward conversion of the α-carbon compounds to enolate ion is attained less favourably with the alkoxide ion when compared to the reverse reaction (note the size of the initial forward reaction in all 3 aromatic esters). Therefore, the enolate ion is required to react with another ester to attain a reaction at equilibrium that goes to completion (Rishan Singh, personal finding).
My discovery while applying the Claisen condensation reaction to these 3 naturally occurring esters, were that β-keto esters are not always the end product of this reaction. I have found that the β-keto ester was only formed in methyl butyrate and not when isopentyl acetate and benzyl acetate is used as the starting material. The very reason for this is that if one looks at the 3 starting carbonyl 1 compounds, it is evident that the R group in methyl butyrate is an ethyl whereas in isopentyl acetate and benzyl acetate it is methyl, therefore when the enolate ion attacks the cation of another ester; it still has two hydrogens in the α-carbon. This means that there is no room for the attachment of a methyl group to the α-carbon. The contrasting view is true for methyl butyrate. This discrepancy doesn’t have any drastic effect or implications on the reaction. It, however, does mean that the Claisen condensation reaction does not always result in a β-keto ester being formed as its end product (Rishan Singh, personal discovery).
Once the O2 atom becomes a negatively charged ester attacked by the enolate ion, a π bond is re-formed between the negatively charged O2 atom and the ‘once-attacked’ C atom (Bruice, 2007). This results in the –OCH3 group being expelled as the leaving group. This is the case for all 3 carbonyl compounds. The alkoxide ion has the ability to reform a tetrahedral intermediate by reacting with the β-keto ester (Bruice, 2007). However, it is unable to react with the enolate ion because the removing of a proton from the starting ester prevents the reverse reaction from occurring because the alkoxide ion, which is negatively charged, will not react with the negatively charged anion of the β-keto ester due to the coulombic (electrostatic) forces of repulsion (Bruice, 2007; Rishan Singh, personal writing).
Essentially, the above is applicable to all 3 aromatic compounds, in addition to the fact that the addition of acid to the reaction mixture has the ability to reprotonate the β-keto ester anion. Furthermore, any remaining alkoxide ion being present in the reaction mixture is also protonated so as to prevent the reaction from shifting to reverse mode (Bruice, 2007).
In conclusion, the Claisen condensation reaction occurs optimally under basic conditions (pH 11 or more), in its aim of obtaining β-keto esters that can be used to synthesis compounds that are of medical and industrial importance. However, the application of the Claisen condensation reaction to methyl butyrate, isopentyl acetate and benzyl acetate, does not reflect this perception since in the reactions involving isopentyl acetate and benzyl acetate, there were no β-keto esters are formed; yet the reaction cannot be said to unsuccessful because the leaving group, CH3O–, clouds that outcome. It can be remarked as a discovery, that the Claisen condensation reaction does not always produce β-keto esters and that the formation of β-keto esters depend on true structure and availability of carbons of the starting ester only (Rishan Singh, personal writing).
References
- Bruice, P.Y. Organic Chemistry (fifth edition), Pearson International Edition, USA, New Jersey: Prentice Hall, Inc, 2007; pp 876 – 877.
- Petrucci, R.H., Harwood, W.S., Herring, F.G (2002). General Chemistry: Principles and modern applications (Eighth Edition), USA, New Jersey: Prentice Hall, Inc, 2002; Glossary A41.

This work is licensed under a Creative Commons Attribution 4.0 International License.









