Synthesis and Characterization of 2-(4-Isopropylbenzyl)-6-Carbamoyl-1, 2-Dihydropurine from 5-Amino-(Cyanoformimidoyl)-1H-Imidazole
A. Yahyazadeh*, S. Porakbari and S. Alizadeh
Department of Chemistry, University of Guilan, P.O. Box 1914, Rasht Iran.
The 5-amino-(cyanoformimidoyl)-1H-imidazole has been prepared from amidine by treatment with barium hydroxide in ethanol. 2-(4-Isopropylbenzyl)-6-carbamoyl-1, 2-dihydropurine was obtained from 5-amino-4-(cyanoformimidoyl)-1H-imidazole and isopropylbenzaldehyde in dry methanol. All compounds have been fully characterized by spectroscopic data.
KEYWORDS:5-amino-4-(cyanoformimidoyl)-1H-imidazole; cyanoimidazole; formamidine; anilinium chloride; imidate; purine
Download this article as:| Copy the following to cite this article: Yahyazadeh A, Porakbari S, Alizadeh S.Synthesis and Characterization of 2-(4-Isopropylbenzyl)-6-Carbamoyl-1, 2-Dihydropurine from 5-Amino-(Cyanoformimidoyl)-1H-Imidazole. Orient J Chem 2008;24(1). |
| Copy the following to cite this URL: Yahyazadeh A, Porakbari S, Alizadeh S.Synthesis and Characterization of 2-(4-Isopropylbenzyl)-6-Carbamoyl-1, 2-Dihydropurine from 5-Amino-(Cyanoformimidoyl)-1H-Imidazole. Orient J Chem 2008;24(1). Available from: http://www.orientjchem.org/?p=23022 |
Introduction
purines are reported to be potent anti-epileptic agents as well as showing anti-anginal and anti- inflammatory activity.1 The most important natural occurrence of purines is in the nucleotides and nucleic acids; compounds which perform some of the most crucial functions in fundamental metabolism. The chemotherapeutic uses of purines and purine analogues have prompted tremendous efforts towards their synthesis, both in academia and in the pharmaceutical industry. 2
Results and Discussion
(Z)-N-(2-Amino-1,2-dicyanovinyl)formamidine (2) was prepared in high yield by reaction of the imidate (1) with ammonia gas at room temperature in the presence of a catalytic amount of anilinium chloride, according to a procedure reported in the literature.3-6
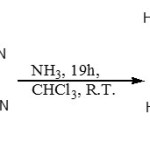 |
Scheme 1: |
Amidine (2) was stored under argon at low temperature in dark since it was found to be unstable on heating.7-9 It was fully characterized and the spectroscopic data obtained were in agreement with those previously reported. The microanalysis results were satisfactory and the low resolution mass spectrum gave a molecular ion peak at m/z 136 [M+1] +. The 1H nmr spectrum showed the presence of the primary amine protons as two broad singlet
at 6.00 and 7.38 ppm and the HC=N proton at d7.72 ppm. In the 13C nmr spectrum there were five peaks as expected, with the cyano carbons at 116.9 and 116.3 ppm. The presences of C≡N groups were also confirmed by bands at 2200 and 2000 cm-1 in the infrared spectrum.
Amidine (2) was then cyclised to 5-amino-4-(cyanoformimidoyl)-1H-imidazole (3) in 78% yield by treatment with barium hydroxide in ethanol.10-12 The reaction was followed by TLC. When all the starting material had been consumed, diethyl ether was added and any base remaining in solution was precipitated as barium carbonate by bubbling CO2 through the solution. Filtration and concentration of the filtrate under reduced pressure (at < 30oC) gave (3) as a pale green solid which was stored under argon at low temperature. It was found that this compound darkens rapidly in solution and decomposes to black oil.
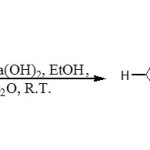 |
Scheme 2: Click here to View Scheme |
The elemental analysis and mass spectroscopy results obtained on this compound (3) were satisfactory. The 1H nmr spectrum showed the presence of three broad singlet a 5.40, 6.55, and 11.78 ppm due to the primary amine, an imine and secondary amine protons and a singlet at 7.28 for the HC proton of the imidazole ring respectively. The 13C nmr spectrum was fully consistent with the assigned structure. The infrared spectrum confirmed the presence of the NH and C=N stretching vibrations within the region of 3400-3100, and 1660-1650 cm-1 respectively. The infrared spectrum also showed a sharp absorption band at 2200 cm-1 for the CºN stretching vibration.
The imidazole derivative (3) was treated then with isopropylbenzaldehyde (4) in dry methanol in a 1:1 molar ratio at room temperature. An orange solid was isolated in 60% yield after 24 hours with the formation of a characteristic red-orange spot on TLC for the 6-carbamoy1-1, 2-dihydropurine derivative.13-16
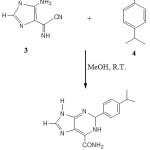 |
Scheme 3: Click here to View Scheme |
The spectroscopic data obtained on this compound suggested that it was the desired 2-(4-isopropylbenzyl-6-carbamoyl-1,2-dihydropurine (5). This was fully characterized by TLC, IR, 1H nmr, 13C nmr and mass spectroscopy. The high resolution mass spectrum gave a molecular ion peak at 282 (M-1)+ which fits with the expected molecular weight of 283 for the dihydropurine (5) and also an accurate mass of 284 due to (M+1)+. The infrared spectrum showed typical N-H stretching vibrations at 3320, 3300, 3260 and 3210 cm-1 and the C=O of the amide group at 1695 cm-1 as a strong band. The H-2 proton appeared as a broad singlet at 4.95 ppm and the four aromatic protons showed the expected patterns in the range of 6.85-6.97 ppm. The H-8 proton of the imidazole ring was seen as a singlet at 7.52 ppm and the NH2 protons as a broad singlet at 7.69-7.85 ppm. However, the NH proton was not detected probably due to the fact that it was too broad and could not be distinguished from the base line.
The 13C nmr spectrum of this dihydropurine had the expected number of peaks and showed broad bands for the carbon atoms participating in the p–system, i.e. C-4, 5, 6 and also CONH2 which appeared on the regions of 170.0 and 176.6 ppm respectively. This broadening has been attributed to tautomersim of the mobile proton between positions N-1 and N-3 as shown below (5a, 5b).2
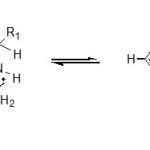 |
Scheme 4: Click here to View Scheme |
Experimental
All solvents purified and dried using established procedures. The 1H NMR spectra were recorded on Hitachi-Perkin-Elmer R24B (60 MHz) or Bruker XL 500 (500 MHz) instruments (with J-values given in Hz), 13C NMR spectra (with DEPT 135) either on a Bruker WP 80 or XL300 instrument, and IR spectra on a Shimadzu IR-470 spectrophotometer. Mass spectra were recorded on a Kratos Concept instrument. The melting points were measured on an Electrothermal digital melting point apparatus and are uncorrected.
References
- Kelly, J.L., Krochmal M.P., Linn J. A. and McLean E. W.: J. Med. Chem., 31, 1005 (1988).
- Alves M. J., Al-Duaij O. Kh., Booth B. L., Carvalho A. Eastwood, P. R.and Proenca M. F. J. R. P.: J. Chem. Soc., Perkin Trans. 1,1705 (1990).
- Booth B. L., Alves M. J., Carvalho A., Eastwood P. R. and Nezhat L.: J. Chem. Soc., Perkin Trans. 2,1949 (1994).
- Elion. G.B., Burg. C. and Hitchings. G.H.: J. Am. Chem. Soc., 74, 411 (1952).
- Yahyazadeh A. and Booth B. L. : Synth. Commun., 23, 3617 (2001).
- Yahyazadeh A. and Booth B. L. : Synth. Commun., 21, 3225 (2001).
- Booth B. L., Alves M.J. and Proenca M. F. J. R. P.: J. Chem. Soc., Perkin Trans. 1, 1705 (1990).
- Sen A. K. and Ray S.: Ind. J. Chem., 14B, 346 (1976).
- Woodward D. W. : U.S. Patent 2 534 331/ 1950.
- Yahyazadeh A. and Sharifi Z.: Phosphorus, Sulfur, and Silicon, 6, 1339 (2006).
- Yahyazadeh A.: Russ. J. Org. Chem., 39, 1718 (2003).
- Yahyazadeh A. and Pourrostam B. : Bull. Korean. Chem. Soc., 24, 1723 (2003).
- Montgomery J. A.: The design of chemotherapeutic agents, Acc. Chem. Res., 19, 293 (1986).
- Yahyazadeh A. and Eslamparast M.: Asian. J. Chemistry, 17, 609 (2005).
- Ostrowski S.; Molecules, 4, 287 (1999).
- Al-Azmi. A. ; Elassar. A. A. and Booth. B. L. Tetrahedron, 59, 2749 (2003).

This work is licensed under a Creative Commons Attribution 4.0 International License.









