Beligand Complexes of Terbium with DTPA-Alanine and Phenlalanine
Abhilasha Asthana, K. Dwivedi and Reetesh Asthana
School of Studies in Chemistry, Jiwaji University, Gwalior - 474 001 India.
The interaction of Tb (III) ions with DTPA alanine and phenlalanine have been followed potentiometrically. The stability constants of the formed complexes are determined at three different ionic strengths.
KEYWORDS:Ternary complex; formation constant
Download this article as:| Copy the following to cite this article: Asthana A, Dwivedi K, Asthana R. Beligand Complexes of Terbium with DTPA-Alanine and Phenlalanine. Orient J Chem 2008;24(1). |
| Copy the following to cite this URL: Asthana A, Dwivedi K, Asthana R. Beligand Complexes of Terbium with DTPA-Alanine and Phenlalanine. Orient J Chem 2008;24(1). Available from: http://www.orientjchem.org/?p=23272 |
Introduction
The studies on mixed ligand complexing equilibria have fascinated the chemists because of the fact such complexes are of much importance in industrial, analytical and biological filed [1-3]. DTPA (Diethelene –triaminepenta acetic acid) is an important octadentate chelating ligand belongs to the class of aminopolycarboxylic acids and find many applications [4-6].
In this paper we have reported the complexes of lanthanide metal ion with DTPA, alanine and phenylalanine.
Experimental Methods
Following sets of titration mixtures were prepared and titrated against standard alkali solution at three different ionic strengths (m = 0.05M, 0.10M and 0.15M) maintained in each set was kept at 50.00ml.
HNO3 ( 2.0×10-3M)
HNO3 ( 2.0×10-3M) + ligand L ( 1.0×10-3M)
HNO3 ( 2.0×10-3M) + ligand L ( 1.0×10-3M) + metal ion ( 1.0×10-3M)
HNO3 ( 2.0×10-3M) + ligand L’ ( 1.0×10-3M)
HNO3 ( 2.0×10-3M) + ligand L’ ( 1.0×10-3M) + metal ion ( 1.0×10-3M)
HNO3 ( 2.0×10-3M) + ligand L ( 1.0×10-3M)+ ligand L’ ( 1.0×10-3M) + Metal ion
Result and Discussion
By plotting the formation cure, pH versus H, the Pk values of the ligand were calculated. These values were in agreement with the literature value. The value of KML can also be calculated considering by Bjerrums function and PL.
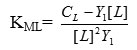
where , CL = Total ligand concentration
L = Ligand
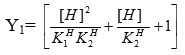
where and are the proton dissociated constant of primary and secondary ligand respectively.
Further the value of formation constant of mixed ligand compelxis calculated by Thomsan and Loraas method. [7]
The plot of moles of alkali per mole of ligand metal (a) vs PH were obtained for the system. It shows studies that the primary complex is formed at a lower PH and is stable even at higher pH value. The primary complex curve [c] and mixed ligand curve [D] overlap each other upto PH »5.2
This indicates that in this PH range combination of secondary ligand with metal ion does not take place. The curve C and D diverse from each other after PH »5.2. At this PH range combination of the secondary ligand with primary complex starts at “a”³ 2, the mixed titration curve is found to be displaced to the right of the theoretical composite curve, which provide the evidence for increased interaction in the presence of two ligand, which indicates the formation of 1:1:1 mixed ligand species. Non appearance of mixed ligand system supports the formation of ternary species.
From above discussion it is concluded that the formation of ternary complex take place by stepwise chelation in which DTPA acts as a primary ligand and alanine and phenylalanine act as a secondary ligand. The equilibria can be represented as
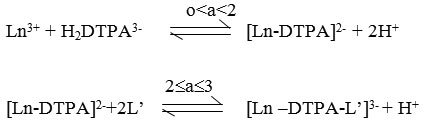
where ,
HL’ = alanine or Phenylalanine
Ln = Tb(III)
Thermodynamic stability constants of complex were obtained graphically by extrapolating to zero ionic strength [m®0]. The graphical extrapolation was obtained by plotting VμVs logK.
The stability order of mixed ligand complees w.r.t secondary ligand is found to be :
alanine < phenylalanine
which is the order of increasing PK value of the secondary ligand.
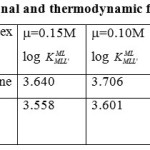 |
Table 1: Conditional and thermodynamic formation constant Click here to View table |
Reference
- Block , S.S.; J. Agri. Food. Chem. 3229 (1995).
- Beck, M.T. and Nagypal, I, Chemistry of complex equilibria, Ellis Herwood limited, Chichester Hastald Press, New York (1990).
- Abu-Baker, M.S.; J. Chem., Technol Biotechnical (1993).
- Kamble, K.K. jatkar, V.S. Tamhankar, S.S., Shahapure, G.D. Damodar, V-; J. Inorg. Nucl. Chem. 42(7) : 1067 (1980).
- Verma, S. and Saxena, M.C., Proc. Indian Acad. Sci., Chem. Sci. 101 (2) : 93 (1989).
- Maharani, A.V. and Arora Neealam, J. Indian Chem. Soc. 60(9) : 833 (1983).
- Thompson, L.C. and Loraas, J.A. Inorg. Chem. 2: 89 ( 1963).

This work is licensed under a Creative Commons Attribution 4.0 International License.









