Abstract
Recent Developments in Weinreb Synthesis and Their Applications
Maher Khalid* , Shireen Mohammed
, Shireen Mohammed and Amin Kalo
and Amin Kalo
DOI : http://dx.doi.org/10.13005/ojc/350601
Abstract:
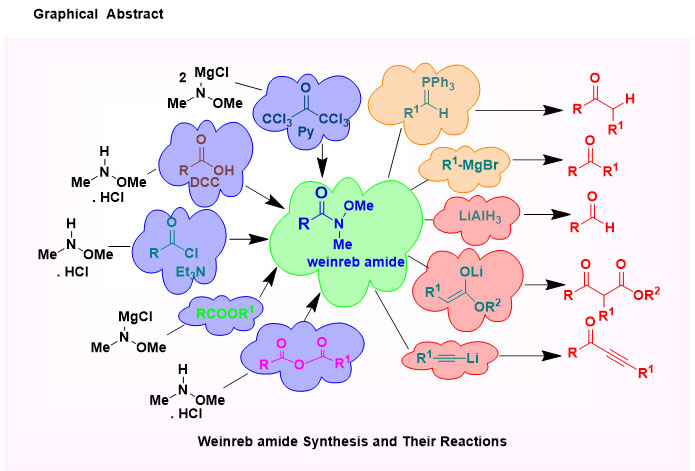
N-methoxy-N-methyl amides or Weinreb amides are worthy embranchment of amide group and their rich functional groups in organic synthesis become a strong else unfeasible conversion. Weinreb amides are produced as an intermediate product of the reaction of carboxylic acids, acid chloride or esters with organometallic reagents, which was first uncovered in 1981. The direct conversion of carboxylic acids or acid chlorides or esters to ketones or aldehydes using organometallic reagents do not lead in high yields, because the intermediate ketones are still highly reactive toward the organometallic reagent. However, after derivatization to the corresponding Weinreb Amide, reaction with organometallics does give the desired ketones, as the initial adduct is stabilized and doesn't undergo further reactions. A nucleophilic addition to the Weinreb amides results in a unique and stable five-membered cyclic tetrahedral intermediate which protects the over-addition, leading to a selective conversion.
N-Methoxy-N-Methyl Amide; Weinreb Amide; Acylating Agents; Asymmetric Hydrogenation; Palladium-Catalyst; C-H Functionalization; Organometallic Reagents
Back to TOC









