Study of the Dissolution of Calcium Carbonate in Different Mixed Solvent at Different Temperatures by Conductometric Method and Data Analysis Using Thermodynamic Parameters
A.K.P.C. Mahavidyalaya, Bengai, Hooghly, West Bengal, India.
Corresponding Author E-mail: hazra.ashoke@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400626
Article Received on : 31 Jul 2024
Article Accepted on :
Article Published : 13 Nov 2024
Reviewed by: Dr Yamin Yasin
Second Review by: Dr. Yash Kushwaha
Final Approval by: Dr. Abdelwahab Omri
Present paper discussed the dissolution behavior of CaCO3 in (water + THF), (water + AN) and (water + DMF) medium at 100C to 300C by conductometric method. From the molar conductance data, we calculate limiting conductance value at infinite dilution using Karus Bray equation. From these data we calculate thermodynamic parameters like ∆H, ∆G, ∆S, ∆Es & Kc. These data helps us to compare dissolution behavior of CaCO3 in different mixed solvent at different temperatures. From this dissolution study of CaCO3 in different non aqueous systems it was observed that stronger ion interaction occur in case of THF and AN & partial dissociation or ion pairing occur in case of DMF solvent. With increase in temperature, limiting molar conductance increases for all the systems. The present work aims to determine the limiting molar conductance values of Calcium Carbonate in different mixed solvents in different temperatures and also evaluate thermodynamic parameters for supporting the ion-solvation interaction.
KEYWORDS:AN (Acetonitrile); Conductance; CaCO3; DMF(Dimethyl Formamide); dissolution; THF(Tetrahydro Furan)
Download this article as:| Copy the following to cite this article: Hazra A. Study of the Dissolution of Calcium Carbonate in Different Mixed Solvent at Different Temperatures by Conductometric Method and Data Analysis Using Thermodynamic Parameters. Orient J Chem 2024;40(6). |
| Copy the following to cite this URL: Hazra A. Study of the Dissolution of Calcium Carbonate in Different Mixed Solvent at Different Temperatures by Conductometric Method and Data Analysis Using Thermodynamic Parameters. Orient J Chem 2024;40(6). Available from: https://bit.ly/3UQcxs8 |
Introduction
Calcium carbonate (CaCO₃) is a common inorganic compound found in rocks, shells, and various biological materials. Its solubility and dissolution behavior are of significant interest in various fields such as geology, environmental science, materials science, and industrial processes. The dissolution process of CaCO₃ varies greatly depending on the solvent used, with notable differences between aqueous and non-aqueous environments.
The dissolution study of CaCO₃ in mixed solvents is essential for a variety of fields, from environmental science to industrial applications. It provides valuable data on how calcium carbonate interacts with different solvent systems, influencing processes like scaling, material degradation, biomineralization, and even carbon sequestration. Understanding these interactions helps in developing strategies to mitigate problems or enhance applications where CaCO₃ is a key component.
In aqueous solutions, the dissolution of CaCO₃ is influenced by the solvent’s polar nature and its ability to stabilize the ions produced during the dissolution process. Water, being a highly polar solvent, can partially dissolve CaCO₃, and this process can be further enhanced by the presence of dissolved carbon dioxide, leading to the formation of carbonic acid. This interaction plays a crucial role in natural processes such as the carbon cycle and the formation of geological features.
Conversely, in non-aqueous solvents, the dissolution behavior of CaCO₃ is markedly different. Non-aqueous solvents, typically characterized by their lower polarity and lack of hydrogen bonding capabilities, generally do not support the dissolution of ionic compounds like CaCO₃. As a result, CaCO₃ remains largely insoluble in these solvents. However, specific conditions, such as the presence of complexing agents or unique solvent properties, can influence the solubility to some extent.
Understanding the dissolution processes of CaCO₃ in both aqueous and non-aqueous solvents is essential for various practical applications and scientific investigations, including water treatment, biomineralization studies, and industrial processes involving calcium compounds. This introduction sets the stage for a detailed exploration of the mechanisms and factors affecting the dissolution of CaCO₃ in different solvent environments.
Dissolution behavior of many salts, weak acids are much interest from many years back 1-4. The solubility of solvents depends on solutes and constituent ions of the solvent mixtures 5-8. CaCO3 is a sparyingly soluble salt, its limiting conductance was not measured by directly Onsagar equation. Here we use Karus Bray equation to determine limiting molar conductance of CaCO3 in different mixed solvent medium. Effect of different salts, sparyingly soluble salts, weak acids in different mixed solvent were studied many researchers 9-11. We already studied the effect of different salts12-13 and weak acids 14-15 in different mixed solvent systems in our laboratory.
The Karus-Bray equation is significant in electrochemistry because it provides a method for determining the limiting molar conductance (ʌ0) of a weak electrolyte, sprayingly soluble salt etc. Limiting molar conductance refers to the conductance of an electrolyte when its concentration approaches zero. This parameter is important because it reflects the intrinsic conductivity of ions.
Our main objective to study to evaluate limiting molar conductance using Karus-Bray equation and also evaluate thermodynamic parameters for supporting the ion-solvation interaction system with this sprayingly soluble salt.
Experimental
Materials
Calcium Carbonate (Merck), THF (Tetrahydro Furan) (PDFCL, Mumbai), AN (Acetonitrile) (LOBA Chemicals ), DMF (Dimethyl Formamide) (LOBA Chemicals) were used as such, without any further purifications.
Solutions preparation
The solution of CaCO3 in water and different compositions (10%, 20%, 40%, 50%, 60%, 80%) were prepared. CaCO3 solution (0.01M) was prepared in water, water + THF, water + AN, water + DMF in volume (v/v) ratio. After that the solution was stirred for 30 minutes and then this solution was kept overnight to get ultimate saturation. The next day the saturated solution of CaCO3 was filtered using Whatman filter paper.
Conductivity study
Conductivity of CaCO3 mixed solvent solutions were measured with a conductivity bridge (EC-TDS analyser, CM-183, Elico). Cell constant varied from 1.0 +10% to 1.0-10% cm inverse. A temperature control bath (made by PDIC, Kolkata) was used to obtain the conductivities at higher temperatures.
Result and Discussion
For this dissolution study we use sprayingly soluble CaCO3. Three solvent systems were taken such as (water +THF), (water +AN) and (water +DMF). Molar conductance of CaCO3 was studied of these three solvent systems by Conductivity Bridge. As CaCO3 is sprayingly soluble salt, so its partial dissociation occurs. So, we can not get limiting molar conductance value from Onsagar equation. To get limiting molar conductance value, we use Karus Bray equation. The equation is
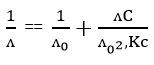
Where ʌ ꞊ molar conductance, ʌ0 ꞊ molar conductance at infinite diluition,
Kc ꞊ Dissociation constant, C ꞊ conc. in mol/dm3
If we plot 1/ʌ vs. ʌC, we get the value of ʌ0 from the intercept. Knowing the value of ʌ0 and C, we can easily calculate Kc from the slope of this plot.
Table 1: Physical properties of Electrolyte solvents
|
Sl No. |
Solvent |
M.W. |
Melting Temp (0C) |
Boiling Temp (0C) |
Viscosity (CP) |
Dielectric Const |
Dipole Moment (D) |
Flash Temp (0C) |
Density (gm/cc) |
|
1 |
THF |
72.10 |
-104.8 |
66 |
0.48 |
7.43 |
1.63 |
-14 |
0.887 |
|
2 |
AN |
41.05 |
-45 |
82 |
0.346 |
35.09 |
3.92 |
2 |
0.786 |
|
3 |
DMF |
73.09 |
-61 |
153 |
0.79 |
37.51 |
3.86 |
67 |
0.944 |
Table 2: Slope value of logʌ vs. logC of CaCO3 with (water + THF), (water + AN) and (water + DMF) medium
|
Sl No. |
Solvent |
Slope value in different temperatures |
||
|
100C |
200C |
300C |
||
|
1 |
(water + THF) |
-0.267 |
-0.348 |
-0.334 |
|
2 |
(water + AN) |
-0.627 |
-0.469 |
-0.448 |
|
3 |
(water + DMF) |
-0.643 |
-0.643 |
-0.686 |
Table 3: Limiting molar conductance value of CaCO3 with (water + THF), (water + AN) and (water + DMF) medium
|
Sl No. |
Temp(K) |
1/T(K-1) |
(water + THF) |
(water + AN) |
(water + DMF) |
|
1 |
283 |
0.0035 |
0.0063 |
0.0173 |
0.0045 |
|
2 |
293 |
0.0034 |
0.0101 |
0.0182 |
0.0055 |
|
3 |
303 |
0.0033 |
0.0104 |
0.0196 |
0.00556 |
Table 4: Dissociation constant, activation of salvation and different thermodynamic parameters of CaCO3 solvated in (water + THF) medium from 283K to 303K.
|
Sl No. |
Temp(K) |
Kc |
∆G (Cal/mole) |
∆S (Cal/mole) |
∆H (Cal/mole) |
∆Es (KCals/mole) |
|
1 |
283 |
5376 |
-5001 |
-23.04 |
-1750 |
4.576 |
|
2 |
293 |
6578 |
-5293 |
-23.24 |
||
|
3 |
303 |
12048 |
-5284 |
-24.85 |
Table 1 shows the physical properties of our experimental non-aqueous solvents (THF, AN & DMF).
Table 2 shows the logʌ vs. logC values of CaCO3 with these three mixed solvent medium. In case of dissolution of CaCO3 in (water + THF) system, slope of logʌ vs. logC at 100C, 200C & 300C is <-0.5. We assume that there was a stronger ion interaction, aggregation, complex electrolyte behavior occurs.
For the dissolution of CaCO3 in (water + AN) system, at 100C, slope value is -0.627 i.e., >-0.5 which indicate ion pairing or partial dissociation occurs, but at 200C & 300C, slope value is <-0.5 . So here stronger ion interaction or aggregation occurs according to Karus Bray equation.
For the dissolution of CaCO3 in (water + DMF) system, slope of logʌ vs. logC at 100C, 200C & 300C we observed that slope value is >-0.5, it can be stated that ion pairing or partial dissociation occurs.
Limiting molar conductances are tabulated in the Table 3 of CaCO3 with these three mixed solvent medium. Limiting molar conductance value increases with increase in temperature for these three mixed solvent medium.
With the help of Karus- Bray equation Kc value can be easily calculated. We know that ∆G ꞊ -RTlnKc, knowing the value of Kc at different mixed solvent medium at different temp, we can easily calculate the value of ∆G. Negative value of ∆G tells us about the spontaneity condition of this dissolution process.
Plot of logKc vs. 1/T, from the slope we can calculate ∆H (change in enthalpy). Using the value of ∆H, we can easily calculate the value of ∆S (change in entropy).
From thermodynamics, we also know that
∆S ꞊ (∆G – ∆H)/T
If we plot log ʌ0 vs. 1/T, from the slope we can easily calculate activation energy for this dissolution process.
From the Table 4, Kc value increases from 100C to 300C of CaCO3 in (water + THF) medium. Negative value of ∆G tells us about the spontaneity condition of this dissolution process. ∆S value also decreases and also tells us the decrease of disorderness. Change in enthalpy value is negative means that the dissolution process is exothermic in nature. ∆Es value is 4.576 K Cals/Mole of CaCO3 in (water + THF) medium.
Table 5: Dissociation constant, activation of salvation and different thermodynamic parameters of CaCO3 solvated in (water + AN) medium from 283K to 303K.
|
Sl No. |
Temp(K) |
Kc |
∆G (Cal/mole) |
∆S (Cal/mole) |
∆H (Cal/mole) |
∆Es (Kcal/mole) |
|
1 |
283 |
1136 |
-4096 |
-18.75 |
-1400 |
0.686 |
|
2 |
293 |
1785 |
-4508 |
-19.49 |
||
|
3 |
303 |
3448 |
-4581 |
-21.13 |
Table 6: Dissociation constant, activation of salvation and different thermodynamic parameters of CaCO3 solvated in (water + DMF) medium from 283K to 303K.
|
Sl No. |
Temp(K) |
Kc |
∆G (Cal/mole) |
∆S (Cal/mole) |
∆H (Cal/mole) |
∆Es (Kcal/mole) |
|
1 |
283 |
4366 |
-4714 |
-22.13 |
-1550 |
2.059 |
|
2 |
293 |
5000 |
-4959 |
-22.21 |
||
|
3 |
303 |
9090 |
-5488 |
-23.22 |
From the Table 5, Kc value increases from low temperature to high temperature. Negative value of ∆H corresponds to the exothermic process of this dissolution of CaCO3 in (water + AN) medium. ∆G value is negative, so spontaneity arises. ∆S value is also negative, dissolution process is not disordered. ∆Es value is 0.686 K Cals/Mole of CaCO3 in (water + AN) medium. ∆Es value is less than the other two medium.
From Table 6, Kc value increase from 100C to 300C for the CaCO3 in (water + DMF) medium. Negative value of ∆G also increases from lower temperature to higher temperature. This means that spontaneity increases at higher temperature. Negative ∆S value also increases from 100C to 300C. This indicates the dissolution of CaCO3 in (water + DMF) medium disorderness increases. Change in enthalpy value is negative means that the dissolution process of CaCO3 in (water + DMF) medium is exothermic in nature. ∆Es value is 2.059 K Cals/Mole of CaCO3 in (water + DMF) medium.
From these three mixed solvent medium, according to ∆Es value CaCO3 in (water + AN) medium is more favourable than the other two medium.
But from other data e.g., Kc, ∆G, ∆S value suggests that CaCO3 in (water + DMF) medium is more favourable than the other two medium.
 |
Figure 1: Plot of 1/ʌ Vs ʌ C of CaCO3 in (water + THF) mixed solvent medium at 100C |
 |
Figure 2: Plot of 1/ʌ Vs ʌC of CaCO3 in (water + THF) mixed solvent medium at 200C |
 |
Figure 3: Plot of 1/ʌ Vs ʌC of CaCO3 in (water + THF) mixed solvent medium at 300C |
 |
Figure 4: Plot of 1/ʌ Vs ʌC of CaCO3 in (water + AN) mixed solvent medium at 100C |
 |
Figure 5: Plot of 1/ʌ Vs ʌC of CaCO3 in (water + AN) mixed solvent medium at 200C |
 |
Figure 6: Plot of 1/ʌ Vs ʌC of CaCO3 in (water + AN) mixed solvent medium at 300C |
 |
Figure 7: Plot of 1/ʌ Vs ʌC of CaCO3 in (water + DMF) mixed solvent medium at 100C |
 |
Figure 8: Plot of 1/ʌ Vs ʌC of CaCO3 in (water + DMF) mixed solvent medium at 200C |
 |
Figure 9: Plot of 1/ʌ Vs ʌC of CaCO3 in (water + DMF) mixed solvent medium at 300C |
 |
Figure 10: Plot of logʌ Vs logC of CaCO3 in (water + THF) mixed solvent medium at 100C |
 |
Figure 11: Plot of logʌ Vs logC of CaCO3 in (water + THF) mixed solvent medium at 200C |
 |
Figure 12: Plot of logʌ Vs logC of CaCO3 in (water + THF) mixed solvent medium at 300C |
 |
Figure 13: Plot of logʌ Vs logC of CaCO3 in (water + AN) mixed solvent medium at 100C |
 |
Figure 14: Plot of logʌ Vs logC of CaCO3 in (water + AN) mixed solvent medium at 200C |
 |
Figure 15: Plot of logʌ Vs logC of CaCO3 in (water + AN) mixed solvent medium at 300C |
 |
Figure 16: Plot of logʌ Vs logC of CaCO3 in (water + DMF) mixed solvent medium at 100C |
 |
Figure 17: Plot of logʌ Vs logC of CaCO3 in (water + DMF) mixed solvent medium at 200C |
 |
Figure 18: Plot of logʌ Vs logC of CaCO3 in (water + DMF) mixed solvent medium at 300C |
Fig.1 to Fig.3 shows the plot of 1/ʌ Vs. ʌC of CaCO3 in (water + THF) in different temperatures. From this plot we can calculate the limiting molar conductance with the help of intercept value.
Fig. 4 to Fig. 6 shows the plot of 1/ʌ Vs. ʌC of CaCO3 in (water + AN) in different temperatures.
Fig. 7 to Fig. 9 shows the plot of 1/ʌ Vs. ʌC of CaCO3 in (water + DMF) in different temperatures.
From these plots we can calculate limiting molar conductance value from the intercept and Kc (dissociation constant) from the slope values. Limiting molar conductance value increases from lower temperature to higher temperatures for these three mixed solvent with CaCO3. Kc values are more increases for CaCO3 with (water + THF) system than CaCO3 with (water + DMF) system. But for the CaCO3 with (water + AN) system Kc values are less increases from lower to higher temperatures.
Fig.10 to Fig.12 shows the Plot of logʌ Vs logC of CaCO3 in (water + THF) mixed solvent medium at 100C to 300C. In case of dissolution of (water + THF) system, slope of logʌ vs. logC at 100C, 200C & 300C is <-0.5. We assume that there was a stronger ion interaction, aggregation, complex electrolyte behavior occurs.
Fig.13 to Fig.15 shows the Plot of logʌ Vs logC of CaCO3 in (water + AN) mixed solvent medium at 100C to 300C. For the dissolution of CaCO3 in (water + AN) system, at 100C, slope value is -0.627 i.e., >-0.5 which indicate ion pairing or partial dissociation occurs, but at 200C & 300C, slope value is <-0.5 . So here stronger ion interaction or aggregation occurs according to Karus Bray equation.
Fig.16 to Fig.18 shows the Plot of logʌ Vs logC of CaCO3 in (water + DMF) mixed solvent medium at 100C to 300C. For the dissolution of CaCO3 in (water + DMF) system, slope of logʌ vs. logC at 100C, 200C & 300C we observed that slope value is >-0.5, it can be stated that ion pairing or partial dissociation occurs.
Plot of logKc vs. 1/T, from the slope we can calculate ∆H (change in enthalpy). Using the value of ∆H, we can easily calculate the value of ∆S (change in entropy).
All the values of ΔG, ΔH and ΔS values are tabulated in Table.
The dissolution data will be analyzed to derive thermodynamic parameters. The Gibbs free energy change (ΔG) will be calculated using the relationship between the equilibrium constant and temperature. Enthalpy (ΔH) and entropy (ΔS) changes will be determined using the van’t Hoff equation, which relates the change in the equilibrium constant with temperature.
Understanding the dissolution of CaCO₃ in mixed non-aqueous solvents has significant implications for various industrial and scientific applications. For instance, in pharmaceuticals, the solubility and stability of active ingredients can be influenced by the solvent environment. In materials science, the interaction of CaCO₃ with organic solvents can impact the fabrication and properties of composite materials. Moreover, this study will contribute to the fundamental knowledge of solubility and thermodynamics in mixed solvent systems, which is critical for designing processes and products in chemical engineering and related fields.
This study aims to bridge the knowledge gap regarding the dissolution of CaCO₃ in non-aqueous solvent systems, providing insights into the thermodynamic parameters governing this process. Through conductometric analysis and temperature variation, this research will offer a comprehensive understanding of how mixed non-aqueous solvents influence the dissolution behavior of CaCO₃, paving the way for future applications and theoretical advancements.
THF is a versatile solvent that dissolves both polar and non-polar compounds relatively well. It is particularly good for dissolving polymers and organic compounds.
AN is highly effective for dissolving polar compounds and salts due to its high polarity. It’s commonly used in high-performance liquid chromatography (HPLC).
DMF is excellent for dissolving a wide range of polar and non-polar substances, including high molecular weight polymers and inorganic salts. It’s often used in organic synthesis and for dissolving difficult-to-dissolve compounds.
Dissolution Power of THF, AN & DMF are different. THF shows good balance between polarity and non-polarity, making it a versatile solvent for many organic compounds and polymers. Not as good as DMF or AN for highly polar compounds or salts. AN (Acetonitrile) was best for highly polar compounds and salts due to its high polarity and less effective for non-polar substances. DMF was the best overall solvent for a wide range of substances, including both polar and non-polar compounds. Its ability to act as both a hydrogen bond donor and acceptor enhances its dissolution power.
This study emphasizes how changes in solvent composition can significantly impact the dissolution behavior of CaCO₃, which is important for optimizing processes in fields such as material science, environmental chemistry, and industrial applications.
Conclusion
For the dissolution of CaCO3 in (water + THF) system, slope of logʌ vs. logC at 100C, 200C & 300C is <-0.5. We assume that there was a stronger ion interaction, aggregation, complex electrolyte behavior occurs.
For the dissolution of CaCO3 in (water + AN) system, at 100C, slope value is -0.627 i.e., >-0.5 which indicate ion pairing or partial dissociation occurs, but at 200C & 300C, slope value is <-0.5 . So here stronger ion interaction or aggregation occurs according to Karus Bray equation.
For the dissolution of CaCO3 in (water + DMF) system, slope of logʌ vs. logC at 100C, 200C & 300C we observed that slope value is >-0.5, it can be stated that ion pairing or partial dissociation occurs.
Kc value increases from 100C to 300C of CaCO3 in (water + THF) medium. Negative value of ∆G tells us about the spontaneity condition of this dissolution process. ∆S value also decreases. Dissolution process is exothermic in nature (∆H=-ve). ∆Es value is 4.576 K Cals/Mole of CaCO3 in (water + THF) medium.
Kc value increases from low temperature to high temperature. Negative value of ∆H corresponds to the exothermic process of this dissolution of CaCO3 in (water + AN) medium. ∆G value is negative, so spontaneity arises. ∆S value is also negative, dissolution process is not disordered. ∆Es value is 0.686 K Cals/Mole of CaCO3 in (water + AN) medium. ∆Es value is less than the other two medium.
Kc value increases from 100C to 300C for the CaCO3 in (water + DMF) medium. Negative value of ∆G also increases from lower temperature to higher temperature. This means that spontaneity increases at higher temperature. Negative ∆S value also increases from 100C to 300C. Change in enthalpy value is negative means that the dissolution process of CaCO3 in (water + DMF) medium is exothermic in nature. ∆Es value is 2.059 K Cals/Mole of CaCO3 in (water + DMF) medium.
From this dissolution study of CaCO3 in different non aqueous systems it was observed that stronger ion interaction occur in case of THF and AN & partial dissociation or ion pairing occur in case of DMF solvent. With increase in temperature, limiting molar conductance increases for all the systems.
Acknowledgement
Author is grateful to the Department of Chemistry, A.K.P.C. Mahavidyalaya for all necessary supports and also thankful to Arnab Hazra , 2nd Year Mechanical Engineering student of NIT Durgapur, West Bengal for drawing necessary plots. Also thankful to Dr. Paramartha Ghosh, Principal for encourage the all types of research work.
References
- Gomaa, E. A., Analele Universitatii den Buchuresti, 2010, 19(1), 45-48.
- Marcus, Y., Preferential salvation in mixed solvents, 1990, 62, 2069-2076.
CrossRef - Gomaa, E. A., Int. Journal of Materials and Chemistry, 2012, 2(1), 16-18.
CrossRef - Perry’s , Chemical Engineering Hand Book, Section 2, Physical and Chemical data, 8 th Edition, Mc Graw Hill, USA(2008).
- Weyl, P. K.; Geochimica et. Cosmochimica Acta, 1959, 17(3-4), 214-225.
CrossRef - Kier, R. S.; Geochim, Cosmochimica Acta, 1980, 44, 241-252.
CrossRef - Millero, F. J.; Geochim, Cosmochimica Acta, 1995, 59, 661-677.
CrossRef - Wallast, R.; Bull Inst. Ocean Monaco Spect., 1994, 13, 13-35.
CrossRef - Marcus, Y.; Pure & Appl Chem, 1990, 62, 2069-2076.
CrossRef - Bhat, J. I., Manjunatha, M.N.; Indian Journal of Chemical Technology, 2003, 17, 462-476.
- Azhar, S.A., Hasan, A.; J. Phys. Chem. Biophys., 2012, 2, 3.
- Hazra, A., Int. Journal of Scientific and Technology Research, 2013, 2(4), 99.
- Hazra, A., Int. Journal of Scientific and Technology Research , 2014, 3(3), 326.
- Hazra, A., Malik, A., Pan, A., Alochana Chakra Journal, 2020, ix(iv), 4269-4285.
- Hazra, A., Orient. J. Chem., 2021, 37(6), 1440-1446.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










