Kinetic Studies on Decolourization of Amaranth by Solar/H2O2 Process
1Department of Chemistry, K. Ramakrishnan College of Engineering (Autonomous),Samayapuram,Trichy-621112,Tamilnadu,India.
2Department of Chemistry, St. Joseph's College (Autonomous, Affiliated to Bharathidasan University, Tiruchirapalli), Tiruchirapalli-620002, Tamil Nadu, India.
3Department of Chemistry, M. Kumarasamy College of Engineering (Autonomous), Karur, Tamilnadu, India.
4Department of Civil Engineering, K.Ramakrishnan College of Technology (Autonomous), Samayapuram, Trichy-621112, Tamilnadu, India.
Corresponding Author E-mail: rajarampraveen85@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380514
Article Received on : 23 Aug 2022
Article Accepted on : 20 Sep 2022
Article Published : 13 Oct 2022
Reviewed by: Dr. Rafal Salam
Second Review by: Dr. Pounraj Thanasekaran
Final Approval by: Dr. Attilio Naccarato
Decolourisation of Anionic cum acidic azo dye Amaranth was carried out by an Advanced Oxidation Process Solar/H2O2. Dye samples of concentration 100 mg/L was taken in 250 ml conical flasks that were treated with 30% H2O2 and exposed to sun light in the intensity range of 60,000-90,000 lux. The impact of various parameters in decolourisation of dye like initial H2O2 concentration, initial dye concentration, initial pH, solar light intensity, additives and temperature was studied in view of kinetics. All the reactions carried out by the impact of system parameters follow pseudo-first order kinetics. Conditions were optimized, and the optimum pH was found to be 12 with an optimum H2O2 concentration of 650 mM to achieve 100% decolourization in 30 minutes. Impact of additives can be studied by adding chloride and dihydrogen phosphate ion of 1 M concentration. Then, mineralization is studied by COD removal. UV-visible spectrometric analysis is done to study degradation level.
KEYWORDS:Azo dye; AOP (Advanced Oxidation Processes); Solar light assisted photodegradation
Download this article as:| Copy the following to cite this article: Raja R, Venis A. R, Padmavathi R, Karthick A. Kinetic Studies on Decolourization of Amaranth by Solar/H2O2 Process. Orient J Chem 2022;38(5). |
| Copy the following to cite this URL: Raja R, Venis A. R, Padmavathi R, Karthick A. Kinetic Studies on Decolourization of Amaranth by Solar/H2O2 Process. Orient J Chem 2022;38(5). Available from: https://bit.ly/3TfliJ5 |
Introduction
Nowadays, there is an increase in the entry of many xenobiotic compounds into the environment because of the increase in production and usage of chemicals in various industries. Dyes are one of those xenobiotic chemical compounds which are required by various chemical-based industries like textiles, paints, food industries, cosmetics, plastics, pharmaceuticals, electronics, paper production, coloring solvents, inks, varnishes, drugs, and leather industries 1-5.
Dyes are chemical compounds with complex aromatic structures, and most of them are highly soluble in water 6. Among them, synthetic azo dyes are found to be the most commonly used commercial textile dyes as they are reported to constitute about 30-40% of the total use. As of now, we know more than two thousand azo dyes 5. They can be identified by the azo groups (-N=N-) that are joined to two organic substituents. 7.
Textile industries are one that release wastewater that is the significant source of entry of dyes into the environment 7-9. A substantial amount of dye is lost in water during the dyeing process, and it is estimated to be around 1-20% of the world’s dye production 2-4. As a result, the textile industry becomes the largest consumer of water, and consequently, it produces a large amount of wastewater leads to water pollution 3-5,7,9,10
Due to the presence of colour producing dyes and other chemicals textile industry wastewaters are distinguished by high TOC and COD values and their release into the aquatic environment affects the photosynthetic activity of aquatic plants and also causes environmental issues like eutrophication and aesthetic pollution 11. Due to the complex structure, reactive azo dyes are non-biodegradable and toxic in a few kinds, mutagenic and carcinogenic, since their products, like amines after the breakdown, have also been found toxic to aquatic lives 2-4,9-12.
Hence, they become a significant concern for shielding the water environment. Hence it is essential from the ecological perspective to remove such dyes from textile industrial wastewater & to treat them so that they do not have any adverse impacts on the environment into which they are released 1,6,9.
The treatments of such organic dyes in wastewater are broadly classified into three types as physical, chemical, and biological treatment methods 1,11,13. The most frequently used physical methods are ultrafiltration and adsorption on activated carbon. The most frequently used chemical methods are coagulation, RO and electrocoagulation. Each method has its own drawbacks 3-5,7-11,12. The treatments such as Aerobic biological treatment and other similar techniques are not enough in breaking down the double bonds of reactive azo dyes [14]. The coagulation process only absorbs them up to some extent, and then the sludge formed from both coagulation and biological processes becomes more problematic to be disposed of 9-11,15.
The above discussed conventional treatment processes are not effective in achieving the complete mineralization of azo dyes. Thus, the elimination or removal of dyes becomes a new challenge 3. Therefore it is necessary to treat these dyes with appropriate methods.
Advanced Oxidation Processes are found to be suitable alternative methods to conventional water treatment processes, as they can decolourise, degrade dyes and other organic water contaminants completely 9,12,13. They are characterized by high efficiency, low cost, and simplicity. 9
Among AOPs, using Hydrogen peroxide in Ultra Violet radiation is a competent method 11. It involves the generation of hydroxyl radicals, which are the most powerful oxidising species after fluorine, that quickly and effectively oxidize a wide range of organic pollutants 51,4,5,7-9,13,15.
The several advantages of UV/ H2O2 treatment are the development of no sludge in the treatment and oxygen formation in the process, which is convenient for other aerobic biological decay processes. Simpler molecules like water, carbon dioxide and simple aliphatic carboxylic acids are obtained as final products by the process 5,7,8,11. The usage of H2O2 has some advantages such as its complete miscibility with water, its commercial availability, no phase transfer problems, and lower investment costs. As a result, H2O2 becomes an environmentally friendly bleaching agent in all kind of industrial wastewater treatments 8-16.
This work’s main objective is to use H2O2 as an oxidizer in presence of sun light for decolourisation of Amaranth dye without usage of any solid catalyst like TiO2, MnO2 and study the degradation in view of kinetics and effects of various system parameters 16.The investigational work defined here assesses the photodegradation of model azo dye Amaranth. Usage of solar radiation and H2O2 without an artificial UV source is also an advantage as solar radiation is abundant in tropical countries like India 4.
Amaranth is a synthetically prepared azo dye that is dark red to purple. It is extensively used in the food and cosmetic industries as the significant coloring agent in cakes and jams 1,14. It is also used in dyeing and colour photography 2. Its usage is banned in many countries due to its toxic nature 1. It is suspected to be carcinogenic and may cause a skin rash. It is recommended to avoid it for people who suffer from asthma or aspirin intolerance 2.
Materials and Methods
Materials Required
Spectrum Private Limited provided the amaranth dye, which was utilized without additional purification.
Experimental procedure
Deionized water was used to prepare amaranth dye solution of 100 mg/L concentration. 250 ml conical flasks were used to prepare the solution that were treated with 30 ml of 30% H2O2, which has 264 mM concentration, and then exposed to sun light with an average intensity of 60,000 to 90,000 lux. The lux meter of model UA 1010B was used to measure the intensity of sun light. Using a digital Spectrophotometer of type Shimadzu1800, the change in the absorbance values of the amaranth dye solution was determined to evidence decolourization.
The UV-Visible spectrometric analysis for dye solutions before and after degradation was also done as a part of the evidence for degradation.
The studies on impact of pH on decolourization of Amaranth was done by treating it with HCl, Citrate buffer, NaOH, Glycine buffer, Phosphate buffer, etc., each of which is of 0.1 M concentration. Dye solution in its own pH was also examined. All the studies were carried out in dye solution of concentration 100 mg/L treated with 30 ml of 30% H2O2, which is of concentration 264 mM. pH ranges from 2-10, and were used for the study. pH was measured by using digital pH meter of model AI-102. After studying the impact of pH in acidic, basic, and neutral conditions, pH was further optimized in basic pH by treating the dye solution with different volumes and different NaOH concentrations in the range 8-12 as its decolourization is more effective in basic pH.
Amaranth was treated with various doses of H2O2 ranging from 100 mM to 1000mM at its optimal pH with dye concentration of 100 mg/L to analyze the impact of H2O2 concentration on the decolourization.
Amaranth was treated with various dye concentrations ranging from 50 mg/L to 500 mg/L in its optimal pH and optimal H2O2 concentration to study the impact of dye concentration on decolourization.
The impact of additives like chloride and dihydrogen phosphate ion on the decolourization of Amaranth dye of 100 mg/L concentration was studied by treating them in concentration of 1 M in its optimal pH and optimal H2O2 concentration.
The impact of solar light intensities on the decolourization of Amaranth dye of 100 mg/L concentration was studied by treating it with different solar light intensities in its optimal pH and optimal H2O2 concentration.
Amaranth dye of 100 mg/L concentration was subjected to decolourisation at various temperatures while being maintained at its optimal pH and optimal H2O2 concentration.
Analytical methods
COD of Amaranth dye before and after degradation by Solar/H2O2 treatment was measured by acid digestion titration method and its reduction is accounted for mineralization.
Kinetic studies on the impact of initial pH, initial H2O2 concentration, initial dye concentration, additives, solar light intensity, and temperature can be done by studying its decolourization at certain time intervals by the decrease in its color. Regarding the dye concentration, the reaction is shown to be of pseudo-first order, which is,
ln [At] – ln [A0] = –kt (1)
in which
k = rate constant of First order
t = Irradiation time
A0 = Initial absorbance value before the start of the decolourization reaction
At = Absorbance value at time t after the start of the decolourization reaction. [17]
 |
Figure 1: Structure of Amaranth. |
Results and Discussion
Impact of initial pH on decolourisation of dye
 |
Figure 2: Impact of initial pH on decolourization of Amaranth dye solution: Dye |
 |
Figure 3: Kinetic studies on impact of initial pH on decolourization of Amaranth dye solution: |
Decolourization of azo dye Amaranth in different pH 2,3,7,9 and 10 was studied by treating it with HCl, citrate buffer, phosphate buffer, glycine buffer. Its decolourization in its own pH 4 was also studied.
It was evidenced that the decolourization in acidic pH 2 was only 57.24%. When pH was increased to 3, it was decreased to 37.82%, and when pH was increased to 4, it was further decreased to 24.5%. In neutral pH 7 decolourization was increased to 40.79%. In basic pH 9 decolourization was increased to 100%. On further increasing pH to 10, decolourization was maintained at 100%. Kinetic studies on the impact of pH have also provided evidence for that. The rate constants calculated for the decolourization reaction in the corresponding pH of 9, and 10 were maximum. Hence it was concluded that maximum decolourization was observed in basic pH of 9 and 10. So it was necessary to further optimize the basic pH from the range 8 to 12.
Decolourization of Amaranth was lower in acidic pH of 2, 3, and 4, and neutral pH of 7 was because of the following reasons:
At pH 7, H2O2 dissociated into the water and oxygen other than forming hydroxyl radical. Hence there will be very low concentration of hydroxyl radicals for attacking the molecules of dye, which results in lower decolourization 18,19.

At pH 3 there was an increase in the concentration of hydroperoxy anions formed from H2O2. Hydroperoxy anion, a conjugate base of H2O2 reacts with H2O2 and reduces the generation of hydroxyl radicals results in lower decolourization 19.

In acidic pH of 2 obtained by the addition of HCl to the dye solution, the concentration of the HCl’s conjugate base, increases, which reacts with radicals, leading to inorganic radicals ions according to the following equation.

These radical ions show a much lower reactivity than hydroxyl radicals. It leads to a decrease in dye decolourization as there arises a rivalry among the anions and dye concerning reaction with hydroxyl radicals 18-20.
Impact of initial basic pH on decolourisation of dye
 |
Figure 4: Impact of initial basic pH on decolourization of Amaranth dye solution. Dye |
 |
Figure 5: Kinetics on impact of initial basic pH on decolourization of Amaranth dye solution. |
On further optimizing the pH for decolourization of azo dye Amaranth in primary condition, it was treated with different NaOH concentrations to get pH ranges from 8 to 12. It was observed that the decolourization was increased from 17.81% to 99.29% on increasing pH from 8 to 9. Decolourization was decreased to 98.66% on further increasing pH from 9 to 10. The decolourization was increased from 98.66 to 99.36% on increasing pH from 10 to 11. The decolourization reached the maximum extent of 100% when pH was increased from 11 to 12. Hence it was concluded that 12 was the optimal pH for decolourization of the dye.
Kinetic studies also proved that. The rate constant calculated for the corresponding pH 12 for decolourization reaction was the maximum.
Tanja Kurbus et al. have reported similar results in the treatment of vinyl sulphone dyes by UV/ H2O2 as all of them had their decolourization in basic pH of 12. A. Riga et al. have reported in the case of degradation of Procion H-excl dyes by UV/ H2O2. Also, the degradation of dyes took place effectively in basic pH of 12 and above. Abbas Rezaee et al. have also reported the same result in reactive blue 19 by UV/ H2O2,which had its effective degradation in basic pH of 11 21-,13.
In basic medium, there is an increase in the concentration of the formation of the conjugate base of H2O2, HO2–. HO2– absorbs solar radiation more powerfully than the H2O2, leading to a rise in the concentration of OH. radicals, resulting in a higher decolourization reaction 18.

Impact of H2O2 concentration on decolourisation of dye
 |
Figure 6: The impact of initial H2O2 concentration on decolourization of Amaranth dye. Dye concentration= 100 mg/L, H2O2 concentration range= 100 mM to 1000 mM, optimal pH=12. |
 |
Figure 7: Kinetic studies on impact of initial H2O2 concentration on decolourization of |
A dye solution with a concentration of 100 mg/L was treated with various concentrations of H2O2 ranging from 100 mM to 1000 mM in its optimal pH 12 to study the impact of initial H2O2 concentration on decolourization of the dye. It was found that 100% decolourization was achieved in the shortest time duration of 0.5 hours (30 minutes) in the H2O2 concentration of 650 mM. Rate constants calculated based on Pseudo-first order kinetics also proved that. Hence it was concluded that 650 mM was the optimized concentration of H2O2.
The lower rate of decolourization of Amaranth dye below and above the optimized concentration of 650 mM was attributed to the following reasons:
H2O2 produces hydroxyl radicals on photolysis reaction that takes place in presence of solar radiations. The radicals formed by the reaction react with excess of H2O2 molecules that forms oxygen and water. Therefore, it leads to lower availability of hydroxyl radicals. Hence the probability of reaction of hydroxyl radicals with dye molecules to degrade them becomes very low. Therefore in a higher concentration of H2O2, the decolourization rate was low 18-24,26.

The generation of hydroxyl radicals was also reduced when H2O2 concentration was decreased. Due to the high concentration of dye molecules, there was an extremely low concentration of hydroxyl radicals to react with and destroy them 18,24-26.
Similar results have been reported by A. Riga et al. in the case of Procion H-excl dyes which were treated by TiO2/UV/ H2O2, Xuanmo Liu in the case of Reactive Black 5 dye treated by Fenton and Fenton-like system, Reza Marandi et al. in the case of Reactive Black B dye treated by UV/H2O2/biosorbent system, Hang Xu et al. in case of Reactive Red SBE treated by Fenton process, Azam Aleboyeh et al. in case of Acid Blue 74 treated by UV/H2O2 process, Fatima H. Al Hamedi et al. in case of Rhodamine B treated by UV/H2O2 process, Silvia Gabriela Schrank et al. in case of Vat Green 01 dye treated by UV/ H2O2 process, Raja et al. in case of Reactive Red-180 by solar/H2O2 process, etc., 17,18,20,20,22,24-26
Impact of initial dye concentration on decolourisation of dye
 |
Figure 8: The impact of initial dye concentration on decolourization of Amaranth |
 |
Figure 9: Kinetic studies on impact of initial dye concentration on decolourization of Amaranth dye: Dye concentration range= 50 mg/L to 500 mg/L concentrations, optimal H2O2 concentration=650 mM, optimal pH=12. |
Treatment of various dye solution concentrations ranging from 50 mg/L to 500 mg/L with the optimal H2O2 concentration of 650 mM at the optimal pH 12 was done to examine the impact of initial dye concentration on the decolourization of Amaranth. It was found that when dye concentration increases, the percentage of decolourization decreases. That was also evidenced by the rate constants determined by Pseudo-first kinetic studies.
The rate of decolourization of Amaranth dye decreases with an increase in dye concentration. It may be because of the following reasons:
The internal absorbance increased as the dye concentration increased, making the dye solution increasingly resistant to solar rays. However, only a specific fraction of the H2O2 in the reaction mixture can be irradiated, which reduces the amount of hydroxyl radicals that are produced. As a result, there was no enough concentration of hydroxyl radicals to react with the surplus dye molecules as the concentration of the dye solution increased and breakdown them. Additionally, the concentration of intermediate molecules, which include highly reactive free radicals, rises. Competition for the ability of dye molecules and intermediate compounds to react with hydroxyl radicals increases. It reduced the bleaching impact of H2O2, which results in a slower rate of decolourization in dye solutions with increasing concentrations 19,24-26,29.
Similar results have been reported by M. Muruganandham et al. in the case of Reactive Yellow 14 treated by UV/H2O2, Xuanmo Li et al. in the case of Reactive Black 5 treated by Fenton and Fenton like system, Hideyuki Katsumata et al in the case of Reactive Yellow 86 treated by the solar-assisted photo-Fenton process, Montaser Y. Ghaly et al. in the case of Maxoline Navy 2 RM 200% basic dye treated by solar/TiO2 process, Amrit Pal Toor et al. in the case of Direct Yellow 12 dye treated by UV/TiO2 process, Hang Xu et al. in the case of Reactive Black B dye treated by UV/ H2O2/biosorbent system, B. Neppolian et al. in case of Reactive Yellow 17, Reactive Red 2, Reactive Blue 4 treated by UV/TiO2 process, Hang Xu et al. in case of Reactive Red SBE treated by Fenton process, Azam Aleboyeh et al. in case of Acid Blue 74 treated by UV/H2O2, Fatima. H. Alhamedi et al. in case of Rhodamine B treated by UV/H2O2, Raja et al. in case of decolourization of Reactive Red 180 dye by solar/H2O2, etc., 17-19,24-31.
Impact of additives on decolourisation of dye
 |
Figure 10: Impact of additives on decolourization of Amaranth: Reaction conditions: (i) Dye solution concentration = 100 mg/L, optimal H2O2 concentration= 650 mM, optimal pH=12. |
 |
Figure 11: Kinetic studies on impact of additives on decolourization of Amaranth: Reaction conditions: (i) Dye solution concentration = 100 mg/L, optimal H2O2 concentration= 650 mM, optimal pH=12. |
Amaranth dye with concentration of 100 mg/L in the optimal pH 12 and optimal H2O2 concentration of 650 mM was treated with additives such as chloride and dihydrogen phosphate of concentration of 1 M to evaluate the impact of them in the decolourization of the dye.
It was observed that both the Chloride ion and Dihydrogen phosphate ion has an enhancement impact on decolourization. The decolourization of dye alone in 20 minutes time duration was only 98.68%, whereas 100% decolourization was achieved by adding Chloride ion and Dihydrogen phosphate ion of 1 M concentration. The rate constants calculated based on kinetic studies also proved that.

In high concentrations like 1 M of Chloride ion, intermediate radical ions may dissociate to form hydroxyl radical, which readily attacks the dye molecules, leading to an increase in dye decolourization reaction, which was not possible in low concentrations like 0.001 M, 0.1 M 32.
Dihydrogen phosphate ion reacts with hydroxyl radical to form intermediate radical ion which acts as radical scavengers. It actually leads to inhibition in decolourization reaction. Similar results were obtained in case of Xiang-Rong Xu et al in case of Orange G degradation by persulphate/Fe2+ process and Miljina D. Radovic et al. in case of decolourization of Reactive Blue 19 by UV/H2O2 process 32,33. But the results obtained show that there was an enhancement in decolourization of Amaranth dye instead of inhibition in decolourization. It may be attributed due to the following reason.
The inhibition impact in decolourization reaction of any dye by both Chloride ion and Dihydrogen phosphate ion occurs in acidic medium and neutral medium. But decolourization of Amaranth took place in a basic medium, and the enhancement by both the ions may be attributed to the fact that generation of more number of hydroxyl radicals from H2O2 in a basic medium, which also observed by Alnuaimi et al. in the case of decolourization of Neutral Red by Photocatalytic TiO2 process 34. Similar results were obtained by Jiangang Qu et al. in the case of Reactive Black 5 dye degraded by UV/H2O2 and Taha M. Elmorsi et al. in the case of Mordant red 73 azo dye degraded by UV/H2O2, which had also had its increase in photodegradation which contains NaCl as an additive in the concentration above 0.2 M. 35,36
Impact of solar light intensity on decolourisation of the dye
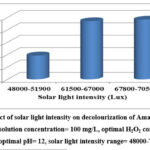 |
Figure 12: Impact of solar light intensity on decolourization of Amaranth: Reaction conditions: Dye solution concentration= 100 mg/L, optimal H2O2 concentration= 650 mM, optimal pH= 12, solar light intensity range= 48000-70500. |
 |
Figure 13: Kinetic studies on impact of solar light intensity on decolourization of Amaranth: |
By comparing the percentage of decolourization of the dye at a concentration of 100 mg/L in various solar light intensities with an optimised concentration of H2O2 at 650 mM and the optimal pH 12, the influence of solar light intensity on decolourization of Amaranth was explored. It was found that an increase in solar light intensity caused an increase in the decolourisation of Amaranth. That was also evidenced by rate constants derived from Pseudo-first order kinetic investigations.
Similar results were observed by M. Muruganandham et al. in Reactive Yellow 14 treated by the solar/ H2O2 method 19.
The increased generation of hydroxyl radicals causes the dye to decolorize more frequently. The quantity of photons absorbed by the H2O2 molecules depends on the intensity of the solar light. H2O2 molecules absorb more photons when the intensity of sun light rises, increasing the amount of hydroxyl radicals. The photolysis of H2O2 in solar/ H2O2 is a direct function of incident light intensity. At low intensity, photolysis of H2O2 produces less hydroxyl radicals, which results in less decolourization, whereas at high intensity, photolysis of H2O2 produces more hydroxyl radicals, which results in more decolourization. All of the photons produced were efficiently employed because it appears that the solar power tested is in the linear range 19,29.
Impact of temperature
 |
Figure 14: Impact of temperature on decolourization of Amaranth: Reaction conditions: Dye solution concentration =100 mg/L, optimal H2O2 concentration= 650 mM, optimal pH= 12, Temperature range= 38-460C |
 |
Figure 15: Kinetic studies on impact of temperature on decolourization of Amaranth: Reaction conditions: Dye solution concentration =100 mg/L, optimal H2O2 concentration= 650 mM, optimal pH= 12, Temperature range= 38-460C |
By comparing the percentage of decolourization of the dye at a concentration of 100 mg/L in different temperatures with the optimal concentration of H2O2 of 650 mM at the optimal pH 12, the impact of temperature on decolourization of amaranth was studied.
It was found that the increase in temperature has led to an increase in the decolourization percentage of Amaranth. Rate constants calculated based on kinetic studies also proved that.
It can be explained on the basis that the decolourisation reaction induced by increase in temperature which leads to an increase in the generation of hydroxyl radicals. Therefore, it enhances the degradation of the dye. This phenomenon can be explained as on increasing temperature up to a certain extent (i.e.) up to 50°C it increases dissociation of H2O2 in the solar-assisted photodegradation process which leads to the generation of more number of hydroxyl radicals. But above 50°C dye degradation decreases because of the decrease in the concentration of Dissolved Oxygen and spontaneous decomposition of hydrogen peroxide into water and oxygen in the dye solution reaction mixture.
Similar results were obtained by Meric et al. in the case of decolourization of Remazol Red dye treated by Fenton process [10] and Pare et al in the case of degradation of Neutral Red by Visible light/ZnO in which 45°C was found at the optimum temperature, in case of decolourization of Reactive Red 120 dye treated by UV/H2O2 by Mansoorian et al. and in case of decolourization of Reactive Blue 19 and Reactive Red 198 treated by Fenton and revised Fenton methods by Rishi Ananth Shankar et al. 37,39
COD removal
 |
Figure 16: COD values of Amaranth dye before and after degradation: Reaction conditions: Dye solution concentration=100 mg/l, optimal H2O2 concentration=650 mM, optimal pH= 12. |
The COD values denote the quantity of oxygen needed for oxidation of substances present in the sample completely by chemical means. The COD values will depend on the dye’s initial concentration, chemical structure, and decolourization time. The COD value of Amaranth dye before degradation was 956 ppm and reduced to 148 ppm after degradation. Hence the COD removal was 84.52%, and it was observed that oxidation was done virtually 21.
It is known that complete mineralization of a dye does not mean that it is completely decomposed into smaller and simpler molecules like CO2, H2O, etc.; there may be some longer-lived reaction intermediates in the degraded and mineralized product. The obtained results were found to be satisfactory and mineralization done by solar/H2O2 occurred effectively. Thus, it was concluded that solar light was more effective in generating hydroxyl radicals by the photolysis process of H2O2. It also activates the bonds of organic molecules to degrade further 21 .
UV-Visible spectrometric analysis
 |
Figure 17: UV-Visible spectrum of Amaranth dye: Before degradation by Solar/H2O2 treatment |
 |
Figure 18: UV-Visible spectrum of Amaranth dye: After degradation by Solar/H2O2 treatment |
The UV-Visible spectrum of Amaranth dye shows four absorption peaks at 521 nm, 375 nm, 331 nm, and 279 nm respectively. Peak at 521 nm corresponds to the Azo group and peaks at 375 nm, 331 nm and 279 nm correspond to aromatic groups like alcohol, naphthalene, sulphonic acid, etc.,after the treatment by solar/H2O2 process, the spectrum shows no absorption peak at all. Hence we conclude that the degradation of Amaranth dye was done effectively.
Rashmi Padhye observed similar biodegradation of Amaranth by the Ligninolytic culture of Phanerochaete Chrysosporium and Eric da Cruz Severo al the case of Amaranth degradation through the photo-Fenton process of heterogeneous by FeWO4 catalyst prepared through microwave irradiation 2-14.
Conclusion
This solar-assisted photodegradation process using H2O2, also known as solar/H2O2, was proved to be an suitable method for decolourization of Amaranth dye of 100 mg/L concentration within the shortest period of 30 minutes completely with the optimum conditions of pH = 12 and H2O2 concentration of 650 mM. UV-Visible spectrometric analysis and Pictorial representation were also provided as a piece of evidence for complete decolourization of dye. COD removal done by this method was also found to be 84.52%. Among the literature surveys done among degradation of Amaranth dye by various methods, it also proved that it was an efficient method as best results obtained among all those. This method’s various advantages are also that it does not form any sludge as formed by Fenton and Photo-Fenton method. It does not form any suspension that has to filter after treatment as formed using TiO2 and ZnO photocatalysts. It is not so harmful as ozone, which is toxic and not so costlier also uses UV light irradiation in combination with H2O2. Hence we can conclude that it is an effective method for the decolourisation and degradation of Azo dyes like Amaranth.
Acknowledgement
The authors thank Test and Best lab private limited for helping in taking UV-Visible spectrum and mineralisation studies(COD removal).
Conflict of Interest
There is no conflict of interest.
Funding Sources
There are no funding Source.
References
- Logamani Pandian; Rajeswari Rajasekaran; Poongodi Govindan. Nanophotocatalytic Ozonation of Textile Dyeing Wastewater using Cu-ZnO Nanocatalyst and Study of Reactor Influencing Parameters.Oriental Journal of Chemistry. ,2019, 35(1), 384-390. (http://dx.doi.org/10.13005/ojc/350148)
- Rashmi Padhye; Raisa Nadaf. Biodegradation of Amaranth by Ligninolytic culture of Phanerochaete Chrysosporium. CIBTech Journal of Biotechnology., 2015,4(4), 30-37. (ISSN: 2319–3859)
- Purnachandar Dachipally; Sreekanth B. Jonnalagadda. Kinetics of ozone-initiated oxidation of textile dye Amaranth in aqueous systems. Journal of Environmental Science and Health., 2011,Part A(46), 887-897. (https://doi.org/10.1080/10934529.2011.580201)
- Yasmin Ali; Aditi Ameta. Degradation and decolourization of Amaranth dye by Photo-Fenton and Fenton reagents: A comparitive study. International Journal of Chemical Science., 2013,11(3) , 1277-1285. (ISSN 0972-768X)
- Hameed, B.H; Lee, T.W. Degradation of malachite green in aqueous solution by Fenton process. Journal of Hazardous Materials., 2009, 164, 468-472. (https://doi.org/10.1016/j.jhazmat.2008.08.018)
- Vitor J.P Vilar; Livia X. Pinho; Ariana M.A. Pintor; Rui A.R. Boaventura. Treatment of textile wastewaters by solar-driven advanced oxidation processes. Solar Energy., 2011, 85, 1927-1934. (https://doi.org/10.1016/j.solener.2011.04.033)
- Behnajady, M.A; Modirshahla, N; Fathi, H. Kinetics of decolourization of an azo dye in UV alone and UV/H2O2 processes. Journal of Hazardous Materials B., 2006,136, 816-821. (http://dx.doi.org/10.1016/j.jhazmat.2006.01.017)
- Azam Aleboyeh; Yasser Moussa; Hamid Aleboyeh. Kinetics of oxidative decolourization of Acid Orange 7 in water by ultraviolet radiation in the presence of hydrogen peroxide. Separation and Purification Technology., 2005, 43, 143-148. (http://dx.doi.org/10.1016/j.seppur.2004.10.014)
- Karami,M.A ; Sharafi,K; Asadi,A; Bagheri,A; Yosefvand,F; Charganeh,S.Sh; Mirzaei,N; Velayati,A. Degradation of Reactive Red 198 (RR198) from aqueous solutions by advanced oxidation processes (AOPS): O3,H2O2/O3 and H2O2/ultrasonic. Bulgarian Chemical Communications., Volume 48, Special Issue D, Supplement, 43-49. (ISSN 08619808)
- Meric,S; Selcuk,H; Gallo,M; Belgiorno,V. Decolourization and detoxifying of Remazol Red dye and its mixture using Fenton’s reagent. Desalination., 2005,173, 239-248. (http://dx.doi.org/10.1016/j.desal.2004.09.002)
- Akyol, A; Bayramoglu, M. Photo catalytic degradation of Remazol Red F3B using ZnO catalyst. Journal of Hazardous Materials., 2005, B124, 241-246. (http://dx.doi.org/10.1016/j.jhazmat.2005.05.006)
- Jatinder Kumar.Photocatalytic Degradation of Amaranth Dye over Immobilized Nano-crystals of TiO2. Recent Advances in Energy & Environment., ISBN:978-960-474-159-5, ISSN:1790-5095, 129-133.
- Eric da Cruz Severo; Chayene Goncalves Anchieta; Victoria Segabinazzi Foletto; Raquel Cristine Kuhn; Gabriela Carvalho Collazzo; Marcio Antonio Mazutti; Edson Luiz Foletto. Degradation of Amaranth azo dye in water by heterogeneous photo-Fenton process using FeWO4 catalyst prepared by microwave irradiation. Water Science & Technology.,2016,73.1, 88-94. (10.2166/wst.2015.469.)
- Mariana Neamtu; Ayfer Yediler; Ilie Siminiceanu; Matei Macoveanu; Antonius Kettrup. Decolourization of disperse red 354 azo dye in water by several oxidation processes – a comparative study. Dyes and Pigments, 2004, 60, 61-68. (http://dx.doi.org/10.1016/S0143-7208(03)00129-3)
- Justina Racyte; Mindaugas Rimeika; Harry Bruning. pH effect on decolourization of raw textile wastewater polluted with reactive dyes by advanced oxidation with UV/H2O2. Environment Protection Engineering, 2009, 35, 167-178. (ISSN: 0324-8828
EISSN: 2450-260X). - Francisco A.P. Costa; Edson M. dos Reis; Julio C.R. Azevedo; Jorge Nozaki. Bleaching and photodegradation of textile dyes by H2O2 and solar or ultraviolet radiation. Solar energy., 2004, 77, 29-35. (http://dx.doi.org/10.1016/j.solener.2004.03.017).
- Fatima H. Al Hamedi; Rauf.M.A; Salman Ashraf,S. Degradation studies on Rhodamine B in the presence of UV/H2O2 , Desalination., 2009, 239, 159-166. (https://doi.org/10.1016/j.desal.2008.03.016)
- Azam Aleboyeh; Yasser Moussa; Hamid Aleboyeh. The Impact of operational parameters on UV/ H2O2 decolourization of Acid Blue 74. Dyes and Pigments., 2005, 66, 129-134. (http://dx.doi.org/10.1016/j.dyepig.2004.09.008)
- Muruganandham, M; Swaminathan, M. Solar driven decolourization of Reactive Yellow 14 by advanced oxidation processes in heterogeneous and homogeneous media. Dyes and Pigments., 2007, 72, 137-143. (https://doi.org/10.1016/j.dyepig.2005.08.009)
- Silvia Gabriela Schrank; Jean Nonato Riberio dos Santos; Danillo Santos Souza; Elayne Emilia Santos Souza. Decolourization effects of Vat Green 01 textile dye and textile wastewater using H2O2/UV process. Journal of Photochemistry and Photobiology A: Chemistry., 2007,186, 125-129. (http://dx.doi.org/10.1016/j.jphotochem.2006.08.001)
- Tanja Kurbus; Alenka Majcen Le Marechal; Darinka Brodnjak Voncina. Comparison of H2O2/UV, H2O2/O3 and H2O2/Fe2+ processes for the decolourization of vinyl sulphone reactive dyes. Dyes and Pigments., 2003, 58, 245-252. (https://doi.org/10.1016/S0143-7208(03)00085-8)
- Riga, A; Soutsas, K ; Ntampegliotis,K; Karayannis,V; Papapolymerou,G. Impact of system parameters and of inorganic salts on the decolourization and degradation of Procion H-exl dyes. Comparison of H2O2/UV, Fenton, UV/Fenton, TiO2/UV and TiO2/UV/H2O2 processes. Desalination.,2007, 211, 72-86. (https://doi.org/10.1016/j.desal.2006.04.082)
- Abbas Rezaee; Mohammad Taghi Ghaneian; Sayed Jamalodin Hashemian; Gholamreza Moussavi, Ali Khavanin; Ghader Ghanizadeh. Decolourization of Reactive Blue 19 Dye from Textile Wastewater by the UV/ H2O2 Process. Journal of Applied Sciences., 2008,8(6), 1108-1112. (https://ui.adsabs.harvard.edu/link_gateway/2008JApSc…8.1108R/ doi:10.3923/jas.2008.1108.1112)
- Reza Marandi; Mohammad Ebrahim Oly; Morteza Khosravi; Rana Khalilnezhad. Decolourization of the Dyes in Aqueous Solution Using a New Combinated System (UV/ H2O2/biosorbent). Journal of Applied Environmental Biological Science., 2013,3(10), 71-80. (ISSN: 2090-4274)
- Montaser Y. Ghaly; Joseph Y. Farah; Amany M. Fathy. Enhancement of decolourization rate and COD removal from dyes containing wastewater by the addition of hydrogen peroxide under solar photocatalytic oxidation. Desalination., 2007, 217, 74-84. (https://doi.org/10.1016/j.desal.2007.01.013)
- Raja, R; Harish Chakravarthi, M; Rose Venis, A; Senthil Kumar, S; Mohandas, T. A study on decolourization of Reactive Red-180 dye and dye effluent containing Reactive Red-180 using H2O2solar assisted photodegradation process. International Journal of Research and Analytical Reviews., 2019,6(2), 818-825. (E ISSN 2348 –1269, PRINT ISSN 2349-5138)
- Xuanmo Liu; Muqing Qiu; Chengcai Huang. Degradation of the Reactive Black 5 by Fenton and Fenton-like system. Procedia Engineering., 2011, 15, 4835-4840. (http://dx.doi.org/10.1016/j.proeng.2011.08.902)
- Hang Xu; Mei Li; Hui Wang; Juan Miao; Lei Zou. Fenton Reagent Oxidation and Decolorizing Reaction Kinetics of Reactive Red SBE. Energy Procedia., 2012,16, 58-64. (https://doi.org/10.1016/j.egypro.2012.01.011)
- Hideyuki Katsumata; Syunya Koike; Satoshi Kaneco; Tohru Suzuki; Kiyohisa Ohta. Degradation of Reactive Yellow 86 with photo-Fenton process driven by solar light. Journal of Environmental Sciences., 2010, 22(9), 1455-1461. (https://doi.org/10.1016/s1001-0742(09)60275-8)
- Amrit Pal Toor; Anoop Verma; C.K. Jotshi; P.K. Bajpai; Vasundhara Singh. Photocatalytic degradation of Direct Yellow 12 dye using UV/TiO2 in a shallow pond slurry reactor. Dyes and Pigments., 2006, 68, 53-60. (http://dx.doi.org/10.1016%2Fj.dyepig.2004.12.009)
- Neppolian,B; Choi,H.C; Sakthivel,S; Banumathi Arabindoo; Murugesan,V. Solar/UV-induced photocatalytic degradation of three commercial dyes, Journal of Hazardous Materials B., 2002, 89, 303-317. (https://doi.org/10.1016/s0304-3894(01)00329-6)
- Miljana D Radovic; Jelena Z Mitrovic; Danijela V Bojic; Milan D Antonijevic; Milos M Kostic; Rada M Baosic; Aleksandar Lj. Bojic. Effects of system parameters and inorganic salts on the photodecolourisation of textile dye Reactive Blue 19 by UV/ H2O2 process. Water SA., 2014, 3(40), 571-577. (http://dx.doi.org/10.4314/wsa.v40i3.21)
- Xiang-Rong Xu; Xiang-Zhong Li. Degradation of azo dye Orange G in aqueous solutions by persulphate with ferrous ion. Separation and purification Technology., 2010,72, 105-111. (https://doi.org/10.1016/j.seppur.2010.01.012)
- Maitha M. Alnuaimi; Rauf,M.A; Salman Ashraf,S. A comparative study of Neutral Red decoloration by photo-Fenton and photocatalytic process. Dyes and Pigments., 2008,76, 332-337. (https://doi.org/10.1016/j.dyepig.2006.08.051)
- Jiangang Qu ; Ruoyang Chen; Xia Dong; Jinxin He. Effect of NaCl concentrations on the photodecoloration of reactive azo-dyes and their cotton dyeings.Textile Research Journal., 2014, 84(20), 2140-2148. (http://dx.doi.org/10.1177/0040517514535867)
- Taha M. Elmorsi; Yasser M. Riyad; Zeinhom H. Mohamed; Hasan M.H. Abd El Bary. Decolourization of Mordant red 73 azo dye in water using H2O2/UV and photo-Fenton treatment. Journal of Hazardous Materials, 2010, 174, 352-358. (https://doi.org/10.1016/j.jhazmat.2009.09.057)
- Brijesh Pare; Pardeep Singh; Jonnalagadda, S.B. Visible light-driven photocatalytic degradation and mineralisation of neutral red dye in a slurry photoreactor. Indian Journal of Chemical Technology, 2010, 17, 391-395. (http://dx.doi.org/10.1080/02726351.2016.1168893)
- Hossain Jafari Mansoorian; Edris Bazrafshan; Ahmadreza Yari; Mostafa Alizadeh. Removal of Azo Dyes From Aqueous Solution Using Fenton and Modified Fenton Processes. Health Scope.,2014, 3(2): e15507, 1-9. (http://dx.doi.org/10.17795/jhealthscope-15507)
- Rishi Ananthashankar; Abdel Ghaly. Effectiveness of photocatalytic decolourization of Reactive Red 120 Dye in textile effluent using UV/H2O2. American Journal of Environmental Science., 2013,9(4) , 322-333. (https://doi.org/10.3844/ajessp.2013.322.333)

This work is licensed under a Creative Commons Attribution 4.0 International License.










