Process of Obtaining Sorbents from Bentonite and Refractory Clays Using Industrial Wastes
Banu A. Userbayeva, Saltanat T. Tleuova, Alibek S. Tleuov, Saltanat D. Arystanova, Marina M. Yeskendirova*, Umirbek P. Baiysbai, Mariam M. Ulbekova and Meruert P. Baiysbaeva
M. Auezov South Kazakhstan State University, Shymkent, Republic of Kazakhstan.
Corresponding Author E-mail: yesmm12@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/350146
Article Received on : 11-10-2018
Article Accepted on : 8-2-2019
Article Published : 26 Feb 2019
The article contains the research results of properties of the sorbents produced from local bentonite clays with addition of oil sludge as a bloating component. To obtain a sorbent with high sorption properties using the local clays and oil sludge the thermal programmed hydrogen reduction and desorption was implemented. The modifying the local bentonite with acid and thermal processing showed, that the sorption properties are improved for account of cation exchange of the elements containing in the initial materials. Calculating Gibbs energy of formation reactions of sorbent minerals in the presence of organic compounds of the oil sludge permitted to conclude that the greatest thermodynamic probability is characteristic for the synthesis of sorbent minerals in the presence of C6H6, and the lowest one - in the presence of CH3Cl. Judging by the results the activated clays containing the oil sludge can be applied for repeated purification of industrial waste water.
KEYWORDS:Aluminosilicate Sorbents; Equilibrium Distribution; Kinetic Regularities; Oil Sludge; Swelling; Thermodynamic Modeling
Download this article as:| Copy the following to cite this article: Userbayeva B. A, Tleuova S. T, Tleuov A. S, Arystanova S. D, Yeskendirova M. M, Baiysbai U. P, Ulbekova M. M, Baiysbaeva M. P. Process of Obtaining Sorbents from Bentonite and Refractory Clays Using Industrial Wastes. Orient J Chem 2019;35(1). |
| Copy the following to cite this URL: Userbayeva B. A, Tleuova S. T, Tleuov A. S, Arystanova S. D, Yeskendirova M. M, Baiysbai U. P, Ulbekova M. M, Baiysbaeva M. P. Process of Obtaining Sorbents from Bentonite and Refractory Clays Using Industrial Wastes. Orient J Chem 2019;35(1). Available from: https://bit.ly/2St2PJg |
Introduction
The absorption regions at 350°C, 650°C, and desorption ones at 433°C were determined by means of a method of hydrogen thermo programmed reduction and desorption. Thermodynamic calculating.1,2 Gibbs energy of possible synthesis conversion of sorbent minerals in the presence of organic compounds of the oil sludge permitted to establish that the greatest thermodynamic probability is characteristic for the synthesis of minerals in the presence of C6H6, and the lowest one – in the presence of CH3Cl.
According to the calculated Gibbs energy the hydrocarbons form a series: CH3Cl>СН4>C2H6>C2H2>С6Н6О>С6Н6. In the presence of С6Н6, the synthesis probability increases in the following series of minerals: Al2O3×SiO2>CaO×SiO2 >3Al2O3×2SiO2>Na2O×CaO∙2SiO2>CaO×Al2O3×2SiO2>CaO×MgO×2SiO2. The physical and mechanical properties of activated bentonite clays with the oil sludge additive of 10-30% are much higher than those of the refractory clays of the Lenger deposit.3–5
The formation process of the porous structure of sorbents, as well as their swelling is affected by the chemical, mineralogical and granulometric compositions of refractory clay rocks.
Research of the materials porosity by means of swelling of the softened mass from within by the gaseous phase contained in the charge or artificially introduced into it and in the presence of oil sludge additives is of particular importance at the development of theoretical foundations of effective technologies for the sorbents production.
Judging by the data of the research of A. Zhukov, V. Sisktor6,7 the gas phase of the pores of the swollen samples contains CO2, H2O, O2, SO2, N2, CO, H2, CH4. In the opinion of these authors, the formation of gaseous compounds is due to the dissociation of carbonates, the reduction of iron oxides, the decomposition of sulfates, water, minerals and impurities. Moreover, the quantitative indicators on the formation of gas phase components are widely spread and give evidence of the need for continuing research in this field.
In this connection, the characteristic reactions of aluminosilicate compounds formation in the presence of hydrocarbon components corresponding to oil sludge were considered to determine the composition of the gas phase and the quantitative distribution of elements.8-10
The determination of formation conditions of aluminosilicate minerals, sorbents, using Darbaza bentonite clays and refractory clays of the Lenger deposit as a main clay component represents interest with reference to the being developed technology. Darbaza clay belongs to the first group and is a good swelling material, which makes it possible to obtain sorbent samples with an apparent density in the piece in the range 0.2-0.5g/cm3 with a swelling coefficient of more than 3-4.5. Lenger clay belongs to the third group and is a clayed weakly-swelling raw material, on the basis of which it is possible to obtain a sorbent with an apparent density of 0.8-1.2 g/cm3 and a swelling ratio lower 2.5-2 (Table 1).
Table 1: Chemical composition of clays (wt.%).
|
Sample name |
SiO2 |
Al2O3 |
Fe2O3 |
TiO2 |
CaO |
MgO |
S |
Na2O |
K2O |
Calcination loss |
| Lenger clay |
59,86 |
20,46 |
6,15 |
0,75 |
1,32 |
1,41 |
0,14 |
0,26 |
2,8 |
6,8 |
| Darbaza clay |
59,0 |
17,29 |
7,76 |
0,75 |
1,19 |
3,0 |
1,85 |
– |
– |
9,1 |
Methodology
The heat treatment of the activated sorbents on the basis of local clays with the oil sludge additive was implemented in a muffle furnace Snol-1300.
The thermodynamic probability of formation of aluminosilicate sorbent minerals in the presence of the organic compounds respective to the oil sludge structure was calculated on the basis of change of Gibbs free energy
![]()
according to the following equation:
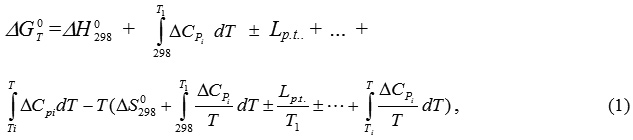
where
![]()
thermal effect of a chemical reaction at 298К equal the difference between the sums of formation heats of products and initial components of the reaction:
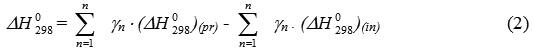
ΔСр – change of thermal capacity of a system equal the difference between the sums of thermal capacities of final and initial substances:
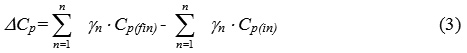
As a rule, thermal capacity of substances depends on temperature according to the following equation:
Ср = a + b × 10-3 × T + c×10-5 × T-2 (4)
To calculate
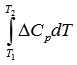
it is necessary to express ΔСр by means of equation 3 as:
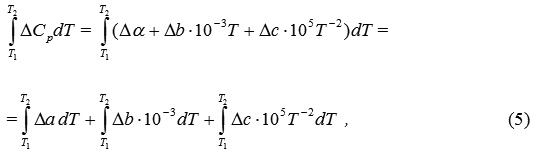
where Δа – the difference between the sums of “a” coefficients of final and initial substances:
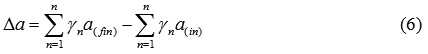
Δb – the difference between the sums of “b” coefficients of final and initial substances:
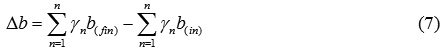
Δc – the difference between the sums of “c” coefficients of final and initial substances:

![]()
entropy of a reaction at 298К equal the difference of the sums of standard entropies of products and initial components of the reaction:
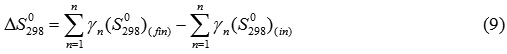
Lp.t. – heat of phase transitions (for products it is used with sign “+” (plus), for initial substances – with sign “–” (minus)).
![]()
was calculated using a software package HSC Chemistry 5.1 of Finnish metallurgical company Outkumpy.
The phase spectrometric and thermographic characteristics of the initial clay components and a bloating additive – oil sludge – were determined with physicochemical methods of analysis.
The X-ray phase analysis of the Lenger and Darbaza clays showed that the main characteristic phases of clays are calcium feldspars with d= 4.21, 4.17Å and potassium feldspars with d = 4.31; 3.31; 3.08; 2.99; 2.97Å. The montmorillonite phase is characterized by diffraction maxima with d = 4.31; 3.92; 2.56; 2.22 Å. The diffraction lines with d = 4.37; 4.31; 2.45; 2.38Å (Fig. 1) are characteristic to the compound Al2O3×3H2O.
Production activities of oil refineries inevitably have technogenic impact on the natural environment. The most dangerous pollutant of all components of the natural environment, surface and groundwater, soil and vegetation cover, atmospheric air is oil-containing waste – oil sludge. Oil sludge in oil refineries is formed in the process of oil refining and wastewater treatment, they are a mixture of precipitations and emulsions detained in sewage treatment plants – sandpipers, oil traps, radial sedimentation tanks, floaters and secondary radial sedimentation tanks. On the toxicity oil sludge is industrial wastes of the third class.
The main quantity of sludge is accounted for by floaters (35-45%) and oil trap (25-30%). Sludge is heavy oil residues, containing on the average (on weight) 56-75% of oil products, 30-85% of water, 1.3-46% of solid impurities, their yield is approximately 0.007 g. per 1 tonne of processed oil.
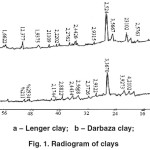 |
Figure 1: X-ray pattern of the clays. |
The main components of oil sludge are oil, oil products contaminated with chemicals, mineralized water and solid suspended substances of various origins. Analysis of the submitted oil sludge sample showed that the content of petroleum products in the upper layers of oil sludge is approximately the same, except for the surface layer enriched with floating oil, an average of 8.1%. The reserves of oil products in all sludge accumulators are about 200-300 tons. The content of mineral impurities in the lower layers is higher than in the upper layers and on average 25-40%. The mineral impurities included in the oil sludge consist mainly of hard sandy and argillaceous particles, industrial dust, coke and coal smut, corrosion products, metal hydroxide particles, alkaline earth metal carbonates, iron carbonates and sulfides, elemental sulfur, and biogrowth of the circulating water supply system. On the basis of mass spectrometric analysis it was established that paraffin-naphthenic, aromatic and resinous components predominate in the hydrocarbon part, with asphaltene inclusions (Table 2).
Table 2: Composition of oil sludge in sludge collectors.
| Collector | Composition, % | |||
| Layer | oil products | mineral impurities | water | |
| Upper | 18,8 | 2,2 | 89,0 | |
| Average | №1 | 24,4 | 4,8 | 70,8 |
| Lower | 16,5 | 40,5 | 43,0 | |
| Upper | 6,7 | 3,3 | 90 | |
| Average | №3 | 25,7 | 11,3 | 60 |
| Lower | 16,8 | 41,4 | 42,8 | |
| Upper | 8,8 | 4,0 | 82,2 | |
| Average | №4 | 26,2 | 8,9 | 64,9 |
| Lower | 18,0 | 40,3 | 41,7 | |
Table 3: Chemical composition of the oil sludge.
|
Title |
Chemical composition, mass % |
|||||||
|
Oil products |
Mineral part |
Moisture |
Coke residue, % |
|||||
|
SiO2 |
CaO |
Fe2O3 |
Al2O3 |
Calcination loss |
||||
|
Oil sludge |
70-75 |
15-20 |
12-20 |
24,8 |
17,3 |
9,47 |
10,9 |
rest |
Table 4: Hydrocarbon composition of the desorbed oil part of the oil sludge.
| Component | Content, % mass |
| Paraffin-naphthene hydrocarbons | 36 |
| Aromatic hydrocarbons | 22,5 |
| Resin | 18,1 |
| Asphaltenes | 8,2 |
| Ash content | 0,4 |
| Sulfur | 0,8 |
| Coking ability | 4,0 |
Differential-thermal analysis of the oil sludge samples was carried out on a derivatograph of the Paulik-Erdei system under the following conditions: heating rate – 50 deg/min; a basic reference standard – calcined aluminium oxide (III); corundum crucibles; temperature range – 293-1273K; the sample weight is 426 mg. At the analysis of the oil sludge sample in the range up to 358K, a weakly expressed endoeffect is observed with the minimum at 328K caused with the weight loss – 1.2%. The further increase in temperature is accompanied by the endoeffect in the range of 358-443K with the minimum at 383K caused with the moisture evaporation. The mass loss in the interval of 358-443K is 38.6%. At Т>443К, the DTA curve is the resultant of superposition of the exo- and endoeffects.
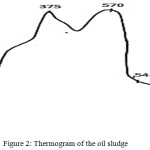 |
Figure 2: Thermogram of the oil sludge. |
IR spectrometric analysis of the oil sludge (Fig. 2) showed that the height of the infrared absorption bands in the region of 600-900 cm-1 explains the presence of the bands characteristic for Si-O-Si valence states. Absorption spectra 1380-1470 cm-1 explain the presence of spectra characteristic for orthoclase, feldspars, which overlap the spectral region up to 1400-1600 cm-1, as well as spectra characteristic of anorthite, albite, and iron-containing microline. Absorption spectra 12-720 cm-1 explain the presence of dolomitized calcite minerals.
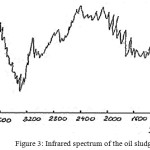 |
Figure 3: Infrared spectrum of the oil sludge. |
To study the hydrogen thermo programmed reduction and desorption the oil sludge samples were placed into a cell, washed with a gas mixture of H2 and Ar (8%) to determine a zero line of a catharometer of a chromatograph “Cromadam”. The gas flow rate is 0.8 ml/s. Then the temperature was linearly increased. The rate of temperature rise was 10°C/min (Fig. 3).
The oil sludge samples were heated to 650°C and kept at this temperature until the achievement of the zero line of 65-70 minutes. After the equalizing the samples in the cell were cooled to the room temperature in a stream of H2 and Ar gas mixture. Then the gas mixture was substituted by an argon flow at rate of 0.8 ml/s and thermal desorption of the adsorbed hydrogen was carried out at the temperature rise rate of 100/min to 650°C.
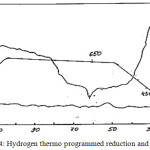 |
Figure 4: Hydrogen thermo programmed reduction and desorption. |
The results of the hydrogen thermo programmed reduction (TPR) of the first sample are characterized by the absorption of H2 at 350°C, then by the liberation, i.e. desorption of H2 at 433°C and absorption of a large amount of hydrogen at 650°C. TPR and desorption of hydrogen from the second oil sludge sample is characterized by two peaks with Tmax = 20°C at the hydrogen rate of 0.4 ml/g and Tmax = 345°C at the hydrogen rate of 0.9 ml/g.
The developed technology for the preparation of sorbents involves using of oil sludge to produce a porous and mechanically strong sorbent.
Thermodynamic studies of the possible formation of minerals of aluminosilicate sorbents in the presence of organic compounds characteristic for the composition of the organic part of the oil sludge were implemented in the temperature interval of 298-1500K. We calculated the Gibb’s energy taking into consideration the changing formation heat (H) and thermal capacity of the components of the reactions. The temperature effect on the thermodynamic probability of sorbent minerals formation with using the oil sludge was studied for 18 reactions in the interval of 298-1500K:
SiO2 + CaO + CH4 + 2O2 = CaO× SiO2 + CO2 + 2H2O (1)
SiO2 + CaO + C6H6 + 7,5O2 = CaO× SiO2 + 6CO2 + 3H2O (2)
SiO2 + CaO + 2CH3CI + 3,5O2 = CaO× SiO2 + 2CO2 + 3H2O + CI2 (3)
SiO2 + CaO + C6H6O + 7O2 = CaO× SiO2 + 6CO2 + 3H2O (4)
SiO2 + CaO + C2H6 + 3,5O2 = CaO× SiO2 + 2CO2 + 3H2O (5)
SiO2 + CaO + C2H2 + 2,5O2 = CaO× SiO2 + 2CO2 + H2O (6)
CaO + Al2O3 + 2SiO2 + C6H6 + 7,5O2 = CaO× Al2O3× 2SiO2 + 6CO2 + 3H2O (7)
CaO + Al2O3 + 2SiO2 + C2H2 + 2,5O2 = CaO× Al2O3× 2SiO2 + 3CO2 + H2O (8)
СaO + Al2O3 + 2SiO2 + C2H6 + 3,5O2 = CaO× Al2O3× 2SiO2 + 2CO2 + 3H2O (9)
CaO + Al2O3 + 2SiO2 + CH4 + 2O2 = CaO× Al2O3× 2SiO2 + CO2 + 2H2O (10)
CaO + Al2O3 + 2SiO2 + 2CH3CI + 3,5O2 = CaO× Al2O3× 2SiO2 + 2CO2 + 3H2O + Cl2 (11)
CaO + Al2O3 + 2SiO2 + C6H6O + 7O2 = CaO× Al2O3× 2SiO2 + 6CO2 + 3H2O (12)
CaO + MgO + SiO2 + CH4+ 2O2 = CaO×MgO×2SiO2 +CO2 +2H2O (13)
CaO + MgO + 2SiO2 +C4H10+ 6,5O2 = CaO×MgO×2SiO2 + 4CO2 + 5H2O (14)
CaO + MgO + SiO2 + C6Н6 +7,5O2 = CaO×MgO×2SiO2 + 6CO2 + 3H2O (15)
Na2O + 2CaO + 3SiO2+ CH4 + 2O2 = Na2O×2CaO×3SiO2 + CО2 + 2H2O (16)
Na2O + 2CaO + 3SiO2+ C4H10 + 6,5O2 = Na2O×2CaO×3SiO2 + 4CО2 + H2O (17)
Na2O + 2CaO + 3SiO2+ C6H6 + 7,5O2 = Na2O×2CaO×3SiO2 + 6CО2 + 3H2O (18)
Curves of changing the heat of formation for the examined reactions in the temperature interval of 298-1500K are represented in Fig. 5 and 6.
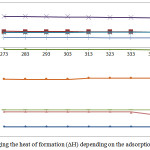 |
Figure 5: Changing the heat of formation (ΔH) depending on the adsorption temperature. |
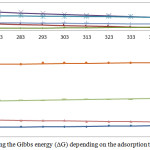 |
Figure 6: Changing the Gibbs energy (ΔG) depending on the adsorption temperature. |
Analysis of the curve of changing the heat of formation for the above mentioned reactions is evidence of positive values of the enthalpy (H) of the reactions 2, 3, 4, 5, 11, 13, 14, 16.
Table 5 contains the summary data concerning the synthesis of minerals of aluminosilicate sorbents (ΔG°т) in the presence of methane, ethane, acetylene, phenol, benzene, chloromethane. At the temperature of 1500K, the Gibbs energy of the reactions involving methane, ethane, acetylene, phenol, benzene, chloromethane is, respectively: –235, –400, –290, –1034, –915, –494 kJ/mol.
Table 5: Temperature and a hydrocarbon type effect on ΔG°т of formation of sorbent minerals.
|
T, K |
DG°т, kJ/mol |
|||||
|
СН4 |
С2Н6 |
С6Н6 |
СН3СI |
C6H6O |
C2H2 |
|
|
Formation of Al2O3×SiO2 |
||||||
| 298 | -933,12 | -1742,88 | -4227,39 | -1501,17 | -1634,75 | -1663,1 |
| 800 | -934,02 | -1776,25 | -6102,58 | -1528,37 | -2082,5 | -2112,6 |
| 1500 | -940,0 | -1840,2 | -8400,3 | -1590,76 | -2790,10 | -2730,2 |
|
Formation of 3Al2O3×2SiO2 |
||||||
| 298 | -996,6 | -1656,53 | +575,25 | -1403,03 | -2995,66 | -1578,0 |
| 800 | -1004,46 | -1708,33 | -1246,51 | -1421,61 | -3005,04 | -2010,4 |
| 1500 | -940,0 | -1800,0 | -3500,0 | -1463,27 | -3131,97 | -2660,0 |
|
Formation of CaO×SiO2 |
||||||
| 298 | -866,32 | -1675,16 | -3244,26 | -2293,13 | -3018,97 | -1291,1 |
| 800 | -868,05 | -1701,53 | -3410,11 | -2156,6 | -3035,82 | -1261,7 |
| 1500 | -871,04 | -1170,24 | -3814,53 | -1839,68 | -3137,3 | -1150,0 |
|
Formation of CaO×Al2O3×2SiO2 |
||||||
| 298 | -978,54 | -1618,76 | -3331,91 | -1862,33 | -1681,35 | -1175,8 |
| 800 | -986,21 | -1647,48 | -3348,65 | -1878,41 | -2066,53 | -1142,7 |
| 1500 | -1002,0 | -1703,97 | -3410,44 | -1927,79 | -2630,2 | -1120,6 |
|
Formation of CaO×MgO×2SiO2 |
||||||
| 298 | -36,82 | -1200,07 | -1128,02 | +366,64 | -1224,6 | +581,4 |
| 800 | -47,13 | -1235,80 | -140,17 | +328,75 | -1256,84 | -21,44 |
| 1500 | -96,88 | -1402,84 | -1193,91 | +303,77 | -1359,51 | -561,28 |
|
Formation of Na2O×CaO∙2SiO2 |
||||||
| 298 | -1014,8 | -1654,65 | -3392,88 | -1570,5 | -3164,25 | -992,16 |
| 800 | -1018,77 | -1672,71 | -3404,34 | -1585,07 | -3204,77 | -921,68 |
| 1500 | -1021,63 | -1716,64 | -3452,37 | -1620,92 | -3291,87 | -921,08 |
The analysis of the obtained results showed that the synthesis of the minerals in the presence of С6Н6 is characterized by the greatest thermodynamic probability and the synthesis in the presence of CH4 – by the lowest one. Hydrocarbons in this case (in accordance with the increase of the reaction probability) form a series: CH3Cl>СН4>C2H6>C2H2>С6Н6О>С6Н6. The formation of Al2O3×SiO2 in the presence of С6Н6 is characterized by the greatest thermodynamic probability, and the formation of CaO×MgO×2SiO2 in the presence of CH4 – by the lowest one. In the presence of С6Н6 the probability of synthesis decreases in the following series of minerals:
Al2O3×SiO2>CaO×SiO2>3Al2O3×2SiO2>Na2O·2CaO·2SiO2>CaO·Al2O3·2SiO2>CaO·MgO·2SiO2
It is known from practice that the swelling value is strongly influenced by various exchangeable cations that are present in the composition of clay minerals. These characteristics of clays are mainly due to the presence of montmorillonite. The cation exchange capacity is explained by the isomorphous replacement of an element with a higher oxidation level by an element with a lower oxidation level and this process is activated with different acid activation.
The acid activation of bentonite clay samples was carried out with 0.1M, 0.5M, 1.8M sulfuric acid. The results of the chemical analysis showed that the content of acidic components, such as SiO2 and Al2O3 increases with the increase in the sulfuric acid molarity. And the content of alkaline and basic oxides, as well as titanium and iron oxides, decreases. These changes are apparently associated with their transition to the sulfate solutions.
Table 6: Chemical composition of activated and non-activated bentonite clay.
|
Component |
Non-activated bentonite clay, % mass |
Bentonite clay activated with sulfuric acid |
||
|
0,1 М |
0,5 М |
1,8 М |
||
|
|
|
Content, % mass |
||
|
SiO2 |
53,61 |
50,2 |
51,3 |
52,3 |
|
Al2O3 |
11,22 |
11,7 |
11,9 |
12 |
|
Fe2O3 |
5,6 |
5,6 |
5,3 |
4,8 |
|
TiO2 |
1,2 |
1,2 |
1,0 |
0,8 |
|
Na2O |
0,63 |
0,4 |
0,33 |
0,2 |
|
K2O |
1,4 |
1,3 |
1,2 |
1,0 |
|
CaO |
2,9 |
2,2 |
1,7 |
1,1 |
|
MgО |
1,0 |
0,9 |
0,6 |
0,5 |
Acid-activated samples of the bentonite clay after flushing were pre-dried to a mass-like state and were mixed with the oil sludge additive in an amount of 10-40%. The mixed compounds were granulated and heat treated at temperatures of 700-1000°C with isothermal aging of 60 minutes. The results of changes in the physico-mechanical properties of the granules heat-treated at the temperature of 1000°C and duration to 60 minutes are given in Table 4. The analysis of the obtained data showed that the physico-mechanical characteristics are improved with the addition of oil sludge up to 30% to the bentonite. At the same time, the mechanical strength reaches 5.22 MPa, the average diameter of the granule is 18.9 mm, the specific surface area is 1560 cm2/g. The physico-mechanical indicators of the sorbents based on the Lenger clays with the additive up to 40% do not differ significantly from the bentonite clays. However, the specific surface and mechanical strength of the sorbents based on the bentonite clays with the addition of the oil sludge is much higher.
Table 7: Physicochemical properties of bentonite sorbents after the heat treatment.
|
Sample |
Density, g/cm3 |
Specific area, cm2/g |
Average diameter of granules, mm |
Strength, МPа |
|
Bentonite |
1,94 |
1323 |
15,7 |
4,3 |
|
Lenger clay |
2,1 |
1345 |
16,3 |
4,2 |
|
Lenger clay + 10% of oil sludge |
2,0 |
1333 |
17,0 |
4,0 |
|
Lenger clay + 20% of oil sludge |
2,2 |
1343 |
18,1 |
4,3 |
|
Lenger clay + 30% of oil sludge |
2,2 |
1349 |
18,3 |
4,33 |
|
Lenger clay + 40% of oil sludge |
2,3 |
1380 |
18,5 |
4,6 |
|
Acid activated bentonite |
2,3 |
1436 |
16,3 |
4,5 |
|
Acid activated bentonite + 10% of oil sludge |
2,4 |
1500 |
18,5 |
5,12 |
|
Acid activated bentonite + 20% of oil sludge |
2,48 |
1530 |
18,6 |
5,2 |
|
Acid activated bentonite + 30% of oil sludge |
2,5 |
1560 |
18,9 |
5,22 |
|
Acid activated bentonite + 40% of oil sludge |
2,5 |
1540 |
18,9 |
5,1 |
Conclusion
The features of chemical composition of the bentonite clays of Darbaza deposit and refractory clays of Lenger deposit are determined on the basis of physico-chemical analyses.
The composition of oil sludge in which paraffin-naphthenic and aromatic compounds dominate in the organic part and compound of the silicon-aluminum with calcium and iron-containing constituents in the mineral part was determined with mass spectrometric and thermogravimetric analysis.
On the basis of the calculated it follows that the synthesis of sorbent minerals in the presence of С6Н6 is characterized by the greatest thermodynamic probability, and the lowest one is characteristic of the synthesis in the presence of CH3Cl. Hydrocarbons in this case (in accordance with the increase of the reaction probability) form a series: CH3Cl>СН4>C2H6>C2H2>С6Н6О>С6Н6;
In the presence of С6Н6 the synthesis probability increases in the following series of minerals:
Al2O3×SiO2 >CaO×SiO2>3Al2O3×2SiO2>Na2O×CaO×3SiO2>CaO×Al2O3×SiO2;
The synthesis of Al2O3×SiO2 in the presence of С6Н6 is characterized by the greatest thermodynamic probability, and the synthesis of CaO∙MgO∙2SiO2 in the presence of CH3Cl –by the lowest one.
The physico-mechanical properties of activated bentonite clays with the addition of oil sludge 10 – 30% are much higher than those indexes of the refractory clays of Lenger deposit.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Su, Ch.; Duan, L.; Donat, F.; Anthony, E. J. Appl. Energy, 2018, 210, 117-126.
CrossRef - Tleuov, A.S.; Tleuova, S.T.; Arystanova, S.D.; Altybayev, Zh.M.; Suigenbayeva, A. Zh. Oriental Journal of Chemistry. 2016, 32(5), 2577-2584.
CrossRef - Soni, V.K.; Sharma, P.R.; Choudhary, G.; Pandey, S.; Sharma, R.K. ACS Sustainable Chem. Eng., 2017, 5(6), 5351–5359.
CrossRef - Cordell, D.; Rosemarin, A.; Schröder, J.J.; Smit, A.L. Chemosphere, 2011, 84, 747-758.
CrossRef - Tleuova, S.T.; Arystanova, S.D.; Bolysbek, A.A.; Baiysbay, O.P.; Abzhanova, A.S. Oriental Journal of Chemistry. 2018, 34(3), 2231-5039.
CrossRef - Cieslik, B.; Konieczka, P. Journal of Cleaner Production, 2017, 142(4), 1728-1740.
CrossRef - Tleuov, A.S.; Altybayev, Zh.M.; Tleuova, S.T.; Sagat, M.S.; Shapalov, Sh.Sh. Reports of the National Academy of Science of the Republic of Kazakhstan, 2016, 2, 89-95.
- Bandura, L.; Woszuk, A.; Kołody, D.; Franus W. Minerals. 2017, 7, 37-45.
CrossRef - Pandey, S. Journal of Molecular Liquids, 2017, 241, 1091-1113.
- Herzel, H.; Krüger, O.; Hermann, L.; Adam, C. Science of The Total Environment. 2016, 542(B), 1136-1143.

This work is licensed under a Creative Commons Attribution 4.0 International License.









