Structural Analysis of LiNixMn2-xO4 Prepared by Irradiation Microwave-Assisted Reflux Technique
Dyah Purwaningsih*, Hari Sutrisno and Haryo Rohmadiyanto
Department of Chemistry Education, Faculty of Mathematics and Natural Sciences, Yogyakarta State University, Jl. Colombo 1, Yogyakarta 55281 Indonesia.
Corresponding Author E-mail: dyah_purwaningsih@uny.ac.id
DOI : http://dx.doi.org/10.13005/ojc/350161
Article Received on : 16-12-2018
Article Accepted on : 06-02-2019
Article Published : 26 Feb 2019
This study aims to synthesize Ni-doped LiNixMn2-xO4 (x = 0, 0.02, 0.04, 0.06, 0.08, 0.1) by irradiation microwave-assisted reflux technique. The microstructure of the products was investigated by x-ray diffraction equipped with U-FIT program package. Results of the structural analysis show that Ni doping changed the size, crystallinity, and micro structure of LiNixMn2-xO4. The LiNixMn2-xO4 solids have a cubic structure with a space group of Fd-3m. Increasing the content of Ni doping does not affect the structure. The average volume of LiNixMn2-xO4 is around 555 Å3 to 580 Å3. The crystallinity of the solids tends to increase with the increasing Ni content accompanied by the decrease in unit cell lattice parameters.
KEYWORDS:Irradiation Microwave-Assisted Reflux Technique; LiNixMn2-xO4; Mole Ratio of Ni/Mn; Microstructure
Download this article as:| Copy the following to cite this article: Purwaningsih D, Sutrisno H, Rohmadiyanto H. Structural Analysis of LiNixMn2-xO4 Prepared by Irradiation Microwave-Assisted Reflux Technique. Orient J Chem 2019;35(1). |
| Copy the following to cite this URL: Purwaningsih D, Sutrisno H, Rohmadiyanto H. Structural Analysis of LiNixMn2-xO4 Prepared by Irradiation Microwave-Assisted Reflux Technique. Orient J Chem 2019;35(1). Available from: https://bit.ly/2GHy6qg |
Introduction
In the current era, innovations in the field of technology have been rapidly increased. One is the electronic portable items such as mobile phones, laptops, cameras, hybrid cars, and others. Battery as a power source was instrumental component in the technological innovation. One type of the power source is a lithium battery. Currently, the common source of lithium is LiCoO2 that widely used as a cathode material for lithium-ion batteries. However, LiCoO2 has several disadvantages which are difficult to be used as a large-sized cathode material, security issues in high power and high cost. This has an impact on the amount of research that focus on the development of other alternative cathode material in view of cost, safety and environment issues.1,2
Although many new cathode materials have been developed, LiMn2O4 is recognized as a promising alternative positive electrode for lithium-ion batteries.3 Spinel structure of LiMn2O4 offers an attractive alternative material beyond LiCoO2 available commercially. The advantages of using LiMn2O4 are that materials are safe, non-toxic, and low cost.4
However, LiMn2O4 has a constraint of life-time, being reduced levels of storage capacity in applications.5 The biggest constraint is the life-time at high temperature. This happens because of the dissolution of manganese into the electrolyte which is then followed by the disproportionation of Mn3+ ions, the decomposition of the electrolyte, and changes in the structure of the powder particles after Jahn-Teller distortion in the cells that consume the battery content.6 These constraints can be reduced by other transition metals having structural conformity to give LiNixMn2-xO4 where M = Co, Mg, Al, Cr, Ni, Fe, Ti and Zn.7
Many methods of synthesis have been reported to prepare LiMn2O4 compound. These includes sol-gel method,8 hydrothermal,9 spray-pyrolysis,10 and so forth. However, studies related to the synthesis of compounds of LiNixMn2-xO4 (x = 0–0.1) are hardly reported, particularly those that deals with the use of irradiation microwave-assisted reflux technique, followed by solid-state reaction.
This paper reports the result of the synthesis of LiNixMn2-xO4 (x = 0–0.1) prepared by irradiation-microwave assisted reflux-technique and its micro structural characterization. By using this technique, it is expected that the complex reactions between lithium ion, nickel ion, manganese ion, and citric acid mono hydrate (as a charge stabilizer and chelating agents) will occur. Irradiation microwave is used so that the collision among the molecules occur more rapidly and therefore the reaction will proceed more easily and efficiently to produce the manganese oxide. Hence, the solid-state reaction was used to lithiation process and doping nickel metal in oxide manganese compounds with the chelating agent. The effects of various mole ratio of Ni/Mn in the synthesis of the products are structurally reported in detail in this work.
Materials and Methods
Synthesis of Mn2O3 and LiNixMn2-xO4
Analytical grade of Mn(CH3COO)2·4H2O and Na2S2O8 (E.Merck) has been used. All other chemicals used without further purification. In a typical synthesis, Mn (CH3COO)2·4H2O and Na2S2O8 with a mole ratio of 1:1 was dissolved at room temperature in 80 mL of deionized distilled water. The mixture was stirred to form a homogeneous solution. The solution was refluxed with the aid of irradiation microwave intensity of 50% of 760 watts for 20 minutes. The precipitate obtained was separated and dried at 250°C for one hour in an oven and calcination at 750°C for two hours.
Synthesis LiNixMn2-xO4 is as follows; one-mole LiOH, 0.02 mol of Ni(CH3COO)2·4H2O, and 1.98 mol of Mn2O3 synthesized, dispersed into citric acid monohydrate to form a thick slurry. It was dried in the oven at 250°C for one hour. Solids of LiNi0.02Mn1.98O4 then was calcined at 750°C for two hours. The same procedure was carried out to synthesize materials with different mole ratios.
Determination and Characterization of LiNixMn2-xO4
Powder Mn2O3 and LiNixMn2-xO4 were analyzed using X-Ray Diffractometer (XRD). The XRD pattern obtained in XRD instrument Rigaku Miniflex 600-Benchtop using Cu Kα radiation (λ = 1.5406 Å) at room temperature. XRD instrument was set to operate at 40 kV and 15 mA. XRD data obtained by 2θ interval ranging from 20°C to 80°C. Rietveld analysis was performed with the software package U-FIT to refine X-ray diffraction data. The parameters for refining are a unit cell, hkl, and volume. SEM image was obtained using a JEOL JSM-6510 LASEM. Effect of nickel content on the structure of LiMn2O4 was studied using Energy Dispersive X-ray (EDX) spectroscopy. EDX analysis is also used to analyze the presence of Mn, O and Ni elements in material prepared.
Results and Discussion
XRD Patterns
Fig 1 shows the XRD generated pattern of LiNixMn2-xO4. High peak intensity indicates that the LiNixMn2-xO4 has a good crystallinity. Fig 2 shows the variation of lattice constants of materials with different compositions. The refinement data obtained using the U-FIT indicates that the lattice parameter decreases with increase in doping content.
Replacement Mn3+ by Ni2+ in the octahedral site (16d) causes a decrease in the lattice parameter, while the lithium ions occupy tetrahedral sites (8a). After modification, lattice shrinkage caused by smaller ionic radius of Ni2+which replaces the Mn3+ sites in 16d. Note that the ionic radius of Ni2+ of 0.560 Å is smaller than that of Mn3+, which is0.645 Å. The differences in the ionic radius between Ni2+ and Mn3+ is relatively small, therefore only a view of changing the lattice parameters is observed. On the other hand, the replacement of Mn3+ by Ni2+ induces the increase of the content of Mn4+ to maintain the balance of the refill process (charge), since Mn4+ has an ionic radius smaller than the Mn3+, this also contribute to lattice shrinkage. Oxygen atoms are arranged in a hermetically sealed packaging 32e.11,12
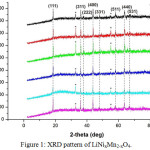 |
Figure 1: XRD pattern of LiNixMn2-xO4. |
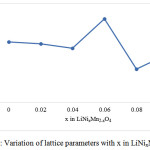 |
Figure 2: Variation of lattice parameters with x in LiNixMn2-xO4. |
Table 1 shows the areas hkl various mole ratio of Ni/Mn in LiNixMn2-xO4 using U-FIT program. The products have a cubic phase with space group Fd-3m as shown by the results of the analysis using the U-FIT program in Table 2. There was no significant difference in the crystal structure after it is being doped. It shows that the Mn sites in LiMn2O4 are replaced completely by Ni, although there are other phases of Mn2O3 are formed. The emergence of this phase of Mn2O3 may be due to the availability of the rest of Mn2O3 which is not completely reacted with LiOH in the lithiation process. This phase is shown by the peak area of 32–33°C and 54–55°C attributed to Mn2O3.
Table 1: Fields hkl of LiNixMn2-xO4.
| x = 0 | x = 0.02 | x = 0.04 | x = 0.06 | x = 0.08 | x = 0.1 | hkl | ||||||
| 2θ | I/Io | 2θ | I/Io | 2θ | I/Io | 2θ | I/Io | 2θ | I/Io | 2θ | I/Io | |
| 18.649 | 100 | 18.463 | 81 | 18.556 | 95 | 18.623 | 87 | 18.591 | 90 | 18.531 | 100 | 111 |
| 35.973 | 87 | 35.884 | 66 | 36.038 | 84 | 36.135 | 77 | 36.112 | 84 | 36.095 | 85 | 311 |
| 37.717 | 7 | – | – | 37.622 | 16 | 37.851 | 30 | 37.837 | 30 | 37.834 | 21 | 222 |
| 43.803 | 96 | 43.705 | 100 | 43.818 | 100 | 43.935 | 94 | 43.912 | 97 | 43.91 | 92 | 400 |
| 47.949 | 23 | 47.88 | 25 | 48.042 | 22 | 48.14 | 32 | 48.098 | 38 | 48.067 | 38 | 331 |
| 58.012 | 30 | 57.967 | 29 | 58.104 | 39 | 58.136 | 31 | 58.136 | 53 | 58.104 | 57 | 511 |
| 63.738 | 56 | 63.705 | 44 | 63.755 | 60 | – | – | 63.928 | 58 | 63.871 | 59 | 440 |
| 67.109 | 14 | 67.004 | 36 | 67.07 | 31 | – | – | 67.203 | 29 | 67.2 | 23 | 531 |
| 75.539 | 4 | 75.307 | 31 | – | – | 75.52 | 23 | – | – | 75.608 | 23 | 533 |
| – | – | – | – | 76.631 | 30 | 76.381 | 15 | 76.767 | 23 | 76.612 | 18 | 622 |
Table 2: Results of analysis using the U-FIT program.
| LiNixMn2-xO4 | Lattice Parameters a = b = c (Å) | Space Group | Crystal Volume (Å3) | Figure of Merite | |
| D | R | ||||
| LiMn2O4 | 8.28383 | Fd-3m | 568.4941 | 0.0237 | 0.0428 |
| LiNi0.02Mn1.98O4 | 8.27991 | Fd-3m | 567.7973 | 0.0466 | 0.0792 |
| LiNi0.04Mn1.96O4 | 8.26919 | Fd-3m | 565.4537 | 0.0294 | 0.0539 |
| LiNi0.06Mn1.94O4 | 8.33935 | Fd-3m | 579.9954 | 0.0307 | 0.0692 |
| LiNi0.08Mn1.92O4 | 8.21942 | Fd-3m | 555.0103 | 0.0187 | 0.0345 |
| LiNi0.1Mn1.9O4 | 8.25553 | Fd-3m | 562.6390 | 0.0224 | 0.0402 |
It seems that lithium ions occupy the tetrahedral (8a) site. After the modification, the lattice shrinkage is due to the smaller ionic radii of Ni2+, which replace the Mn3+ at 16d sites. On the other hand, Ni2+ substituting part of Mn3+ can enhance the content of Mn4+ to keep the charge in balance, and Mn4+ is smaller ionic radii than that of Mn3+.12 Oxygen atoms are arranged in the cubic-closed packing 32e.
SEM-EDX Images
Fig 3 shows the SEM-EDX Mn2O3, LiMn2O4, and the LiNi0.08Mn1.92O4. Based on the SEM image it can be seen that in general the three samples showing the non- homogeneous particle surface. Qualitatively, it appears that the particles forms agglomeration. It is indicated by the structure of particles that joined by each other compatibly, so that the grain/single particles do not appear clearly. It is noteworthy that the shape of the particle is basically irregular cubic.
The average particle size of the nickel-doped samples is smaller than that of undoped LiMn2O4. The doping of LiNixMn2-xO4 with Ni causes the tendency of nucleation process and hinder the formation of crystal growth. The Ni-doped LiMn2O4 powders have irregular particle size. The increase in the nickel doping leads to increase in LiMn2O4 particle size. The morphology is almost similar.11
As expected, the EDX data of the materials show the Ni content in accordance with the predetermined Ni to Mn molar ratio. It suggests that the Ni content in the LiMn2O4 corresponds to the amount of Mn in the structure that has been replaced. Also, it is also clearly observed the peak intensity of Ni increases with an increase in Ni content, as expected.
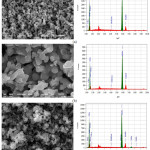 |
Figure 3: SEM-EDX of (a) Mn2O3 (b) LiMn2O4 and (c) the LiNi0.08 Mn1.92O4. |
Conclusion
It has been shown that LiNix Mn2-xO4 has successfully been synthesized by using irradiation microwave-assisted reflux technique. The LiNixMn2-xO4 powders have a cubic crystal structure with an Fd-3m space group. The particle size of the prepared materials is not homogeneous. Doping with Ni leads to change in size, crystallinity, and microstructure of the product. The average volume of LiNixMn2-xO4 is about 555 Å3 to 580 Å3. The crystallinity of the materials tends to increase with the increase in the Ni doping content. On the other hand, the lattice parameter decreases with the increase of Ni content. The synthesized LiNixMn2-xO4 has been expected to give a better performance as a lithium-ion battery’s cathode.
Acknowledgements
The authors thank the Ministry of Research, Technology and Higher Education of the Republic of Indonesia for the financial support through the Hibah Bersaing Program.
References
- Chebiam, R.V.; Prado, F; Manthiram, A.; Chem. Matter., 2001, 13, 2951-2957.
CrossRef - Venkatraman, S.; Shin, Y.; Manthiram, A.; Electrochem. Solid-State Lett., 2003, 6, A9-A12.
- Fergus, J. W.; J. Power Sources, 2010, 195, 939-954.
CrossRef - Julien, C. M.; Mauger, A.; Zaghib, K.; Groult, H., Inorganics, 2014, 2, 132-154.
CrossRef - Hu, M.; Pang, X.; Zhou, Z., J. Power Sources, 2013, 237, 229-242.
CrossRef - Gabrisch, H.; Ozawa, Y.; Yazami, R., Electrochim. Acta, 2006, 52, 1499-1506.
CrossRef - Peng, C., Bai, H., Xiang, M., Su, C.; Liu, G.; Guo, J., Int. J. Electrochem. Sci., 2014, 9, 1791-1798.
- Michalska, M.; Lipińska, L.; Mirkowska, M.; Aksienionek, M., Diduszko, R.; Wasiucionek, M., Solid State Ionics, 2011, 188, 160-164.
CrossRef - Yue, H. J.; Huang, X. K.; Lv, D. P.;Yang, Y., Electrochim. Acta, 2009, 54, 5363–5367.
CrossRef - Iriyama, Y.; Tachibana, Y.; Sasasoka, R.; Kuwata, N.; Abe, T.; Inaba, M.; Tasaka, A.; Kikuchi, A.; Kawamura, J.; Ogumi, Z., J. Power Sources, 2007, 174, 1057-1062.
CrossRef - Purwaningsih, D.; Roto, R.; Sutrisno, H., IOP Conf., 2014, 107, 1-8.
- Li, X.; Xu, Y.; Wang, C., J. Alloys Compd., 2009, 479, 310-313.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









