Nanophotocatalytic Ozonation of Textile Dyeing Wastewater Using Cu-Zno Nanocatalyst and Study of Reactor Influencing Parameters
Logamani Pandian1, Rajeswari Rajasekaran*2 and Poongodi Govindan3
and Poongodi Govindan3
1Research and Development Centre, Bharathiar University, Coimbatore-641046, India.
2Department of Chemistry, Quaid-E-Millath Govt. College for Women, Chennai-600002, India.
3Department of Physics, Quaid-E-Millath Govt. College for Women, Chennai-600002, India.
Corresponding Author E-mail: rajikanna99@yahoo.co.in
DOI : http://dx.doi.org/10.13005/ojc/350148
Article Received on : 12-12-2018
Article Accepted on : 09-01-2019
Article Published : 09 Feb 2019
Nanophotocatalytic ozonation (PCO) of textile dyeing wastewater with Cu-ZnO nanocatalyst was studied in a laboratory scale recirculating photocatalytic ozonation batch reactor. A bench scale reactor was fabricated to suit the integration of ozonation and nano photocatalytic treatment. After optimizing the reaction parameters such as Ozone dosage, Catalyst dosage, pH for nano PCO with Copper doped Zinc Oxide (Cu-ZnO) nanocatalyst and Amaranth dye as a model pollutant, the efficiency of the PCO reactor was investigated with respect to ozone dosage (0.24g/h-0.57g/h), liquid volume (1000mL-5000mL), recycle rate (6 L/h- 39L/h). Based on the optimized parameters, the mineralization of the Amaranth dye resulted in 85.6% removal. Ozone dose was found to have a significant impact on the process efficiency. Wastewater from textile dyeing industry showed 95% degradation measured by COD (Chemical Oxygen Demand) removal and 80% mineralization by reduction of TOC. (Total Organic Carbon). Biodegradability of the treated wastewater increased to 0.42 implying that the further treatment could be progressed by biological method. This study on recirculating batch reactor shows that this treatment could be scaled up for industrial sector.
KEYWORDS:Batch Reactor; Cu-ZnO; Mineralization; Nano Photocatalytic Ozonation; Textile Dyeing Wastewater
Download this article as:| Copy the following to cite this article: Pandian L, Rajasekaran R, Govindan P. Nanophotocatalytic Ozonation of Textile Dyeing Wastewater Using Cu-Zno Nanocatalyst and Study of Reactor Influencing Parameters. Orient J Chem 2019;35(1). |
| Copy the following to cite this URL: Pandian L, Rajasekaran R, Govindan P. Nanophotocatalytic Ozonation of Textile Dyeing Wastewater Using Cu-Zno Nanocatalyst and Study of Reactor Influencing Parameters. Orient J Chem 2019;35(1). Available from: https://bit.ly/2DqCfep |
Introduction
Water the most essential component for all the living and non living organisms present in the world has become a costlier commodity nowadays due to shrinking of the water resources and hence protecting the integrity of our water resources is the burning environmental issues of the 21st century. Since the release of industrial wastes into the water bodies results in the increase of the environmental risk, the mankind’s goal should be at least to treat such compounds in a way that they do not have adverse impacts on the environment into which they are released. In general, the elimination of organic pollutants in waste water needs one or more basic treatment techniques that are broadly classified into physical, chemical and biological treatment processes. Depending on the pollutant present, the treatment methods can be destructive or non-destructive. The choice of the method basically depends on the cost of the process, the concentration and volume flow of the effluent to be treated. Since trace recalcitrant compounds such as pesticides, surfactants and dyes in the wastewaters are resistant to the conventional chemical and biological treatments, Advanced Oxidation Processes (AOPs) are being studied as an alternative to traditional methods and will probably constitute the best option in the near future.
In general, a combination of several methods gives high treatment efficiency compared with individual treatment. AOPs are based on the generation of very reactive species such as hydroxyl radicals (OH•) that oxidize a broad range of organic pollutants quickly and non-selectively.1 Consequently, integration of two or more AOPs enhances free radical generation, which eventually leads to higher oxidation rates.2 Even though ozonation and photocatalysis have been studied for variety of pollutants, the degradation required longer treatment time and complete mineralization was rarely achieved. But when these two methods are integrated, due to synergistic effect of ozone on the photocatalyst, recombination of electrons and holes are prevented and large amount of powerful hydroxyl radicals are produced. As an innovative water treatment, Photocatalysis integrated with ozonation as Photocatalytic ozonation (PCO) is used for degradation of wide variety of pollutants.3-6 Among the various photocatalysts available in the field, Zinc oxide shows potential because of its high chemical stability, catalytic activity, low cost and non toxic nature. However, the rapid recombination rate of the photo induced charge carriers in ZnO results in reduction of its photocatalytic activity which makes it unsuitable for commercial application.7 This recombination of charge carriers could be reduced by doping which creates more defects and vacancies which trap charge carrier and enhance the photocatalytic activity.8-10 Recent studies demonstrated that transition metal doping into ZnO lattices can tune various properties of ZnO and enhance its visible light photocatalytic activity.11-14 Studies have been reported in which the doped catalysts generally act better than their pristine counterparts. Hence for the present study, Copper doped Zinc oxide (Cu-ZnO) is synthesized and used for PCO. The mechanism outlying the synergistic effect of ozone on nanocatalyst is shown in figure 1 where more hydroxyl radicals (oxidants) generated enhances the degradation of pollutants is clearly depicted.
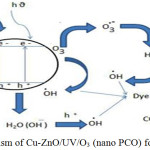 |
Figure 1: Mechanism of Cu-ZnO/UV/O3 (nano PCO) for degradation of dye. |
As PCO appears to be the most promising technology for the degradation of waste waters, promoting this to real industrial scale treatment needs to be studied. Investigations in actual wastewater from industry containing the mixture of organics is very limited therefore, the present study aims at designing a bench scale reactor for the treatment of textile dyeing waste waters and study of the influencing parameters. The development of bench scale reactor is a complex exercise, since it has to deal with catalyst in the solid phase and ozone in the gaseous phase and the pollutants in the liquid phase. The configuration of the reactor must be able to address the important parameters namely the efficient exposure of photocatalyst to UV radiation, a good contact among the pollutant, nano photocatalyst, ozone and the effective utilization of ozone with minimum amount of unreacted ozone leaving the reactor. Based on the above concepts, an attempt is made to fabricate a bench scale reactor where both the nano photocatalyst and light was effectively utilized.
Although various industries are responsible for contributing these hazardous organic wastes into our water resources, the textile industry is of major concern. Because of their persistence and potential adverse health effects, presence of dyes in aquatic systems has been recognized as a major issue in many countries and hence wastewater from textile dyeing industry has been taken for the present study.
In the present work, the effects of the operating parameters such as ozone dosage, liquid volume and recycle rate on the efficiency of nano PCO in the batch reactor were investigated with Amaranth dye as a model pollutant and then its applicability to real textile dyeing wastewater was also studied. Amaranth is an commonly used Azo dye in textile industry and is major colouring agent in cakes and jam. Due to its toxic nature, its usage is banned in many countries.15,16 The objective of this study is to reduce the total organic content of the wastewater and make it biodegradable in nature so as to couple with biological treatment.
Materials and Methods
Materials
Commercial grade Amaranth (Chemical name: trisodium (4E) -3-oxo-4-[(e-sulphonato-1-napthyl)hydrazono] naphthalene-2,7-diphonate) which is commonly used in textiles and also as food colouring additive was obtained from local dye suppliers. All the other chemicals such as Zinc acetate dehydrate, Copper (II) acetate monohydrate, NH4OH, H2SO4 KI, Na2S2O3 5H2O, and NaOH were AR grade purchased from Merck. All the aqueous solutions are prepared with double distilled water. Textile dyeing wastewater was collected from a small scale dyeing industry before the treatment.
Bench scale photoreactor design
Preliminary experiments on nano PCO such as optimization of catalyst dosage, ozone dosage and pH were performed with synthesized Cu-ZnO nanocatalyst in an annular photocatalytic ozonation reactor with 500ml of dye solution.17 The process is now scaled up in a Bench Scale photocatalytic ozonation reactor for 5 litres of wastewater. The PCO reactor comprised five parts namely the Ozone generator, UV panel, Storage tank or reactor tank, circulating pump and KI traps. The reactor tank is made of plexiglass box and connected by PVC tubes. For all the experiments, this system was operated in a recirculation mode. The reactor had an inlet and outlet for feeding and collecting the effluent samples. O3 gas was purged into the reactor from the bottom. The excess or unreacted O3 from the reactor after the reaction was collected from the top of the reactor and allowed into buffered KI traps to measure the amount of unreacted ozone and then in to fume hood. The whole set up was placed inside a plywood box which housed three UV lamps (8 W, Philips, 365 nm) at the bottom side of the top cover. The illumination unit was aligned in parallel to reactor surface at a distance of 5 cm. The plywood box was also provided with two exhaust fans to maintain the temperature inside the reactor setup. The schematic representation and actual picture of bench scale reactor set up is shown in Figure 2 and 3 respectively. Ozone was generated by passing air into ozone generator (Indiozone model, India) and the excess unreacted ozone was let into KI traps. Ozone input flow rate was adjusted with a flow meter on the generator and hence desired dosage could be passed to the reactor. A peristaltic pump provided the flow of wastewater into the reactor.
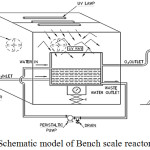 |
Figure 2: Schematic model of Bench scale reactor for nano PCO. |
 |
Figure 3: Bench scale reactor setup. |
Experimental Procedure
The performance of the reactor was evaluated by varying the parameters such as applied ozone dosage, liquid volume and recycle rate of the dyeing wastewater. Simulated wastewater was pumped into the reactor by peristaltic pump with different recycle rates and irradiated by UV lamp at the top of the reactor. Ozone was continuously bubbled into the reactor through a porous diffuser at the bottom. The ozone dose could be adjusted by changing the input flow rate. The reactor was filled with 5000 mL of dyeing wastewater after adjusting the pH to 6 and 0.1 g/L of Cu-ZnO nanocatalyst was mixed to make a suspension. Ozone was bubbled into reactor and the degradation achieved at different time intervals was studied by withdrawing the samples from the outlet at the side of reactor. The amount of unreacted ozone was measured from the KI traps by iodometric titrations. The utilization of ozone at different reaction conditions was calculated based on which optimum reaction conditions for attaining maximum degradation of wastewater was arrived.
Analysis
Textile dyeing wastewater collected from industry was analysed as per the standard procedures for the determination of basic characteristics. Absorption peaks corresponding to Amaranth showed a linear dependence with initial concentration. The concentration of dye was thus monitored by measuring the absorbance on a Spikol 1200 UV-Vis spectrophotometer at 522nm wavelength. The decrease in absorption maximum was observed with increasing reaction time for the degradation of Amaranth. TOC determination was carried out with TOC 5000 Shimadzu TOC analyzer. BOD and COD analysis were performed as per standard procedure.18
Results and Discussion
Effect of Ozone Dosage
To determine the effect of ozone dose on degradation efficiency of PCO in the bench scale reactor, a series of experiments were carried out by varying the ozone dosage from 0.28 g/h to 0.57 g/h for duration of 6 h with a recycle rate of 18 L/h. It was found that ozone dose significantly affected the degradation efficiency and as the ozone dose increased, higher COD removal is anticipated. But when the ozone dosage increased from 0.24 g/h to 0.57 g/h the COD removal increased upto 0.44 g/h above which decrease was observed as shown in the figure 4. It was observed that at 0.44 g/h and 0.57 g/h the COD removal was 90.0% and 82.7% respectively. This might be due to the loss of ozone at the high concentration prior to the oxidation of organics. At high dosage, the contact of dye molecules with ozone molecules was decreased, resulting in the faster release of unreacted ozone. This was established by measuring the ozone in the off gas by collecting in KI traps and analyzing by iodometric titration. Similar results have been obtained by other researchers.3,19 The ozone utilization and COD removal achieved by nano PCO is listed in table 1. Even though the utilization of ozone was higher at 0.24 g/h and decreased at higher dosages, based on the degradation efficiency for the present study 0.44 g/h was selected as optimum dosage at which maximum degradation with effective utilization of ozone was observed.
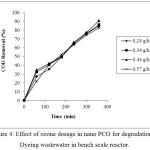 |
Figure 4: Effect of ozone dosage in nano PCO for degradation of Dyeing wastewater in bench scale reactor. |
Effect of Liquid Volume
The effect of liquid volume on the degradation was investigated at three different liquid volumes (1000 mL, 3000 mL and 5000 mL) with the same ozone dosage 0.44 g/h. It was observed from the results that the degradation efficiency decreased slightly with increase in liquid volume which might be due to the less penetration of the light. But with increase in volume of the wastewater, utilization of ozone increased and at about 84% utilization was observed with 5000 mL of wastewater. The effect of liquid volume on the performance of the bench scale reactor and the COD removal achieved is shown in figure 5.
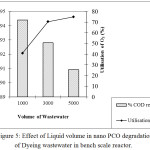 |
Figure 5: Effect of Liquid volume in nano PCO degradation of Dyeing wastewater in bench scale reactor. |
Table 1: Utilisation of Ozone at different Ozone dosages in Bench Scale Reactor.
| S.No. | Parameter | Simulated dyeing wastewater fed with different ozone dosage | |||
| 1 | Ozone dosage (g/h) | 0.242 | 0.342 | 0.448 | 0.572 |
| 2 | Ozone Reacted (g/h) | 0.212 | 0.28 | 0.378 | 0.304 |
| 3 | Ozone Unreacted(g/h) | 0.03 | 0.058 | 0.07 | 0.268 |
| 4 | Utilisation of Ozone % | 87.6 | 83.04 | 84.3 | 53.1 |
| 5 | Degradation efficiency % | 84.6 | 86.2 | 90.9 | 82.7 |
Effect of Recycle Rate
The effect of recycle rate of wastewater on degradation by nano PCO was investigated by varying the recycle rate at 6 L/h, 18 L/h and 39 L/h. Dyeing wastewater was bubbled with ozonized air from the ozonator at a rate of 0.44 g/h with 0.1 g/L of Cu-ZnO nanocatalyst dispersed in the solution and the results are shown in figure 6. It was found that the recycle rate had negligible influence on the degradation efficiency. The degradation at 6 L/h and 18 L/h was better than that obtained at 39 L/h. This might be due to increase in thickness of the liquid film and less photons react on the nano photocatalyst surface resulting in the slower production of hydroxyl radicals and consequently less photocatalytic reaction rate.20 Further as recycle rate is increased, the contact time of the effluent on photocatalyst and with ozone is decreased, and this in turn reduced the rate at which the organic molecules react with photogenerated holes or hydroxyl radicals. Zou and Zhu20 investigated the effect of recycle rate on the colour removal of reused water by photocatalytic ozonation and reported that colour removal achieved at lower flow rate (0.3 and 0.6 L/min) was better than obtained at higher flow rate (1.2 L/min).
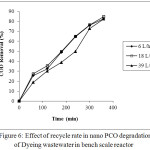 |
Figure 6: Effect of recycle rate in nano PCO degradation of Dyeing wastewater in bench scale reactor. |
Decomposition and Organic Carbon removal of dyeing wastewater by nano PCO
Bench scale studies were performed with 365 nm lamp which did not necessitate the use of Quartz envelope and thus reducing the cost of the reactor. By regulating the ozone dose, effective utilization of ozone was achieved with maximum degradation of pollutant. Studies were conducted with 5000 mL of dyeing wastewater containing 40 mg/L of Amaranth dye, 0.1 g/L Cu-ZnO with ozone dosage of 0.44 g/h and at pH 6 with the recycle rate 18 L/h. The figure 7 depicts the COD removal, TOC removal and inorganic ions formed during degradation process. The concentration of dye reduced from 40.5 mg/L to 1.5 mg/L at the end of 360 min of nano PCO. The evolution of nitrate ions during the degradation process indicated that the dye has disintegrated with the evolution of inorganic ions.
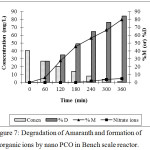 |
Figure 7: Degradation of Amaranth and formation of inorganic ions by nano PCO in Bench scale reactor. |
Application of Integrated Nano PCO to Real Wastewaters from Textile Industry
The efficacy of PCO was studied for wastewater from textile dyeing industry. The effluent was collected from a dyeing industry and characterized. The untreated effluent was found to have pH 8 and high concentration of organics which was indicated by high COD value. The bench scale reactor was fed with 5000 mL of wastewater from industry along with Cu-ZnO (0.1 g/L) after adjusting the pH to 6. The wastewater was agitated in the dark for 60 min for effective mixing of the catalyst and the pollutant. Ozone was then fed into the reactor at a flow rate 0.44 g/h and the irradiation was provided by the UV lamps placed at the top of the reactor. The samples were withdrawn at specified intervals of time and analysed. Table 2 shows the characteristics of the real wastewater before and after nano PCO treatment. The change in COD, TOC and BOD with respect to time is shown in figure 8. The results indicate that at the end of 480 min 95% COD removal and 80% TOC removal was achieved. Further, the biodegradability of the wastewater has improved from 0.10 to 0.42 which indicates the possibility of biological treatment as a tertiary step of degradation.
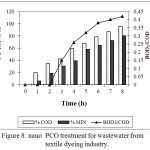 |
Figure 8: nano PCO treatment for wastewater from textile dyeing industry. |
Table 2: Characteristics of textile dyeing effluent before and after photocatalytic ozonation treatment.
| S.No. | Parameter |
Initial value |
Final value |
| 1 | pH |
8.0 |
6.5 |
| 2 | Colour |
Turbid |
Colourless |
| 3 | Chloride (mg/L) |
850 |
1200 |
| 4 | Phosphate (mg/L) |
650 |
1400 |
| 5 | Sulphate (mg/L) |
640 |
1200 |
| 6 | Nitrate (mg/L) |
250 |
650 |
| 7 | COD (mg/L) |
1400 |
65 |
| 8 | TOC (mg/L) |
284 |
56 |
| 9 | BOD (mg/L) |
150 |
27 |
Table 2 indicates that the amount of inorganic ions formed after the treatment has increased which might be due to the release of ions from fragmentation of dyes and also from the additives or formulating agents which were added during the dyeing process. The colour of the effluent turned from turbid to colourless. Removal of the organic matter was indicated by the reduction in COD and TOC values and increase of BOD3/COD to 0.42 after the treatment shows that the wastewater could be further treated by biological method.
Conclusion
The results from Bench Scale reactor study also showed that the reactor could be scaled up for industrial application with effective utilization of ozone and light source at lower cost for the degradation of different dyes and additives present in the industrial effluent. The suggested treatment process has increased the biodegradability and achieved 95.4 % degradation of the textile dyeing wastewaters. With effective stimulation of nanophotocatalyst Cu-ZnO by O3/UV, this study on PCO shows that it can be effectively applied to degrade textile industry wastewaters with proper reactor design.
References
- Gogate, P.R; Pandit, A.R.; Adv. Environ. Res, 2004, 8, 501-551.
CrossRef - Audenaert ,W.T; Vandierendonck, D; Van Hulle, S.W; Nopens, I; Water Res. 2013, 47(7), 2387–2398.
CrossRef - Li, L; Zhu, W; Chen, L; Zhang, P; Chen, Z; J. of Photochem. Photobiol. A: Chemistry., 2005, 175, 172-177.
CrossRef - Mano, T.; Nishimoto, S.; Kameshima, Y.; Miyake, M;. Journal of the Ceramic Society of Japan, 2011, 119(11), 822–827.
CrossRef - Mena, E.; Rey, A.; Contreras, S.; Beltrán, F. J;, Catalysis Today, 2014, 252, 100–106.
CrossRef - Parrino, F.; Camera-Roda, G.; Loddo, V.; Augugliaro, V.; Palmisano, L.; Applied Catalysis B: Environmental, 2015, 178, 37–43.
CrossRef - Udo, M.K; Ram, E.K; Stefanakos, A.F; Hepp, D; Yogi G; Mater. Sci. Semicond. Process., 2013, 16, 2070-2083.
CrossRef - Manjula G. Nair; Nirmala, M; Rekha, K; Anukaliani, A; Mater. Lett., 2011, 65, 1797–1800.
CrossRef - Timonah N. S; Yang C; Sun L; Sci. Adv. Mater, 2010, 2, 534-538.
CrossRef - Aisah N; Gustiono D; Fauzia V; Sugihartono I; Nuryadi R; Materials Science and Engineering, 2017, 172, 012037.
- Rekha, K; Nirmala, M; Manjula G. Nair; A. Anukaliani; Physica B, 2010, 405, 3180–3185.
CrossRef - Muruganandham, M; Zhang, Y; Suri, R; Lee, G.J; Chen, P.K; Hsieh, S.H; Sillanpaa, M; Wu, J.J; Journal of Nanoscience and Nanotechnology, 2015, 15(9), 6900-6913.
CrossRef - Jongnavakit, P; Amornpitoksuk, P; Suwanboon, S; Ndiege, N; Appl. Surf. Sci., 2012, 258, 8192–8198.
CrossRef - Zhao, J; Wang, L; Yan, X.Q; Yang, Y; Lei, Y; Zhou, J; Huang, Y.H; Gu, Y.S; Zhang, Y; Mater. Res. Bull. 2011, 46, 1207–1210.
CrossRef - Divya, N; Bansal A; Jana, A.K; Mater. Sci. Forum, 2012, 712, 85–104.
CrossRef - Elgendy, E.M; Al-Zahrani, N.A.A; Indian J. Sci. Res., 2015, 4, 827–832.
- Logamani P, Rajeswari R, Poongodi G; Materials Research Express, 2018, 115505.
- APHA, Standard methods for the examination of water and wastewater, 21st edition. American Public Health Association, Washington DC, USA. 2005,
- Kerc,A.; Bekbolet,M; Saatci,A.M.; Ozone Sci. Engg., 2003, 25(6), 497-504.
CrossRef - Zou L; Zhu, B;. J. Photochem. Photobiol. A: Chemistry, 2008, 196(1), 24-32.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









