Synthesis of Schiff bases Derivatives of 1,3-oxazepine and Evaluation of Antioxidant Action In vitro
Sarvesh Kumar Singh* , Neelima Mishra, Govind Nayak
, Neelima Mishra, Govind Nayak and Parulben Mehta
and Parulben Mehta
Lakshmi Narain College of Pharmacy, Bhopal, Madhya Pradesh, India.
Corresponding Author E-mail: sarveshsingh14121@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390534
Article Received on : 09 Sep 2023
Article Accepted on : 11 Oct 2023
Article Published : 17 Oct 2023
Reviewed by: Dr. Ayesha Khan
Second Review by: Dr. Sunil
Final Approval by: Dr. Ayssar Nahle
The objective of this work was to synthesize new 1,3-oxazepine derivatives and evaluate the antioxidant activity of the synthesized compounds. The synthesis of Schiff’s base of oxazepine was achieved in four steps involving condensation of aniline and benzaldehyde followed by (2+5) cycloaddition occurs between the imine and anhydride leading to the formation of 1,3-oxazepine-4,7-dione. Later the oxazepine dione undergoes condensation with thiosemicarbazide and finally nucleophilic addition of active carbonyl group to form Schiff’s bases. The synthesized compounds 8a-e were obtained in yield ranging from 69-77%. The antioxidant activity of the compounds was evaluated at various concentrations (100-500µg/mL) using DPPH radical scavenging assay and hydroxy radical scavenging assay. The compounds had IC50 in the range of 34.297 µg to 131.04 µg in the DPPH scavenging assay whereas the IC50 ranged of 49.943 µg to 153.13 µg in the hydroxy radical scavenging assay.
KEYWORDS:Antioxidant; DPPH; hydroxy radical; 1,3-oxazepine; Schiff’s base,
Download this article as:| Copy the following to cite this article: Singh S. K, Mishra N, Nayak G, Mehta P. Synthesis of Schiff bases Derivatives of 1,3-oxazepine and Evaluation of Antioxidant Action In vitro. Orient J Chem 2023;39(5). |
| Copy the following to cite this URL: Singh S. K, Mishra N, Nayak G, Mehta P. Synthesis of Schiff bases Derivatives of 1,3-oxazepine and Evaluation of Antioxidant Action In vitro. Orient J Chem 2023;39(5). Available from: https://bit.ly/3Q289Dj |
Introduction
Oxazepine are seven membered heterocyclic structures with one nitrogen and one oxygen replacing the carbon atom at various positions 1. . Three isomeric forms of oxazepine have been known (Figure 1).
 |
Figure 1: Isomeric forms of oxazepine |
The most common route of synthesis of oxazepine involves pericyclic cycloaddition of Schiff base or hydrazone with maleic, phthalic and succinic anhydrides2-6. Oxazepine derivatives were found to exhibit a vast variety of biological activities like antibacterial 7, antifungal 8, hypnotic muscle relaxant 9, antagonistic 10, inflammatory 11 and antiepileptic 12.
Chronic disorders such cancer, diabetes, neurodegenerative diseases, and cardiovascular diseases all have oxidative stress as a major contributor to their aetiology 13. In some biological systems, reactive oxygen species (ROS) are created as part of regular cellular oxygen metabolism 14. Oxidative stress may develop as a result of the impact of an increase in ROS on intracellular antioxidant capacity 14. Free radicals including hydroxyl and superoxide anion radicals as well as nonradical species like singlet oxygen and hydrogen peroxide are examples of ROS 15. ROS cause oxidative stress and affect hepatic and extrahepatic organs. Degenerative diseases like cancer, dementia, and ageing are thereby caused by leadership, either directly or indirectly 16.
Antioxidants are necessary substances that lower or neutralise ROS, shielding organisms from ROS and shielding cells from oxidative damage. As a result, a lot of study has been done to find newly synthesised antioxidants to stop damage caused by ROS 17.
Recent research have shown that oxazepines and their derivatives are used as chemical inhibitors of enzyme activity in clinical, crucial pharmaceutical, and synthetic chemistry applications. The antibacterial, antiradical, antioxidant, antiepileptic, antihyperglycemic, antimicrobial, antidote, anti-carcinogenic, and anti-HIV properties of oxazepines were previously described.
Considering the vast potential of oxazepine heterocycles, in the present work we attempted to synthesize new 1,3-oxazepine derivatives and evaluate the antioxidant activity of the synthesized compounds.
Material and Methods
Aniline, benzaldehyde (SD Fine), maleic anhydride, 4-chlorobenzaldehyde, 4-hydroxybenzaldehyde (Loba), 2-nitrobenzaldehyde, 3-nitrobenzaldehyde (Sulab) and thiosemicarbazide (CDH) were used in the study. All the other solvents were procured form Sigma, Finar and Qualigens and were of analytical grade. Precoated silica gel G plates were used for thin layer chromatography and distilled water was prepared fresh using glass distillation assembly. Melting point of the compounds was checked using melting point apparatus by open capillary procedure and remain uncorrected for atmospheric variations.
The scheme for synthesis of the Schiff bases of oxazepine (Scheme 1) was devised using the methods reported in various literature 18-20. The methods were modified as per the requirement of the reactants to obtain product in high yields.
 |
Scheme 1: Pathway for synthesis of compounds |
Synthesis of benzylidene benzenamine, 3
In a 25 mL beaker accurately measured benzaldehyde (10 mmol) and aniline (10 mmol) were mixed with 10 mL of ethanol at room temperature and 2-3 drops of glacial acetic acid was added to it. The mixture was then exposed to microwave irradiation at 270W for 2-3 min. The reaction mixture was then cooled in ice bath the products purified by recrystallization in ethanol to give the product 3 18.
Synthesis of 1,3-oxazepine-4,7-dione, 5
Maleic anhydride (10 mmol) in toluene (10 mL) was added to product 3(10 mmol) in toluene (20ml). The reaction mixture was refluxed for 2 h. The solvent was removed under reduced pressure using rotary evaporator. The solid product was recrystallized using ethanol to obtain 5 of sufficient purity 18.
Synthesis of thiosemicarbazide derivative of oxazepine, 6
Equimolar amounts of thiosemicarbazide and oxazepine dione were combined with 5 (10 mmol), 20 mL of ethanol, and 8–10 drops of glacial acetic acid. After 6 hours of heating at 90 °C, the reaction mixture was cooled to 20 °C. The following crude was filtered, methanol washed, and dried 19.
General procedure for synthesis of Schiff base of oxazepine, 8a-e
A solution of aryl aldehyde (0.001 mol) in absolute ethanol (20 ml) and a few drops of glacial acetic acid were combined with a solution of oxazepin-thiosemicarbazone, 6, (0.001 mol) in absolute ethanol (30 ml). For 12 hours, the stirred reaction mixture was refluxed. A precipitate formed after cooling, which was filtered out, rinsed with cold ethanol, and then re-crystallized from ethanol 20.
Antioxidant Assay
DPPH Scavenging Assay
The antioxidant action of the synthesized compounds was determined using 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay 21.
Utilising the stable radical DPPH, the synthetic compounds’ free radical scavenging activity was assessed in terms of their capacity to donate hydrogen or to scavenge free radicals. The test samples (100 mL, 100–500 g/mL) were made in DMSO and then filled with methanol to a final volume of 4 mL before being combined with 1.0 mL of DPPH solution. In a visible spectrophotometer, the resulting solution’s absorbance was measured at 517 nm. Ascorbic acid served as the benchmark substance. Higher free radical scavenging activity was shown by the reaction mixture’s lower absorbance. The percentage of free radicals that the sample successfully inhibited was used to express the radical scavenging activity and was computed using the formula below:

where At is the absorbance in the presence of the test samples and Ao is the absorbance of the control (blank, without sample). The results of each test were run in triplicate, and mean values minus standard deviations were used to express the findings.
Hydroxy radical scavenging activity
Test solutions of different concentrations (100 L)—100, 200, 300, 400, and 500 g/mL—were added to with 1 mL of iron EDTA solution, 0.5 mL of EDTA solution, 1 mL of DMSO, and 0.5 mL of ascorbic acid. For 15 minutes, the mixture was heated to between 80 and 90 degrees in a boiling water bath. After 15 minutes of room temperature incubation, the reaction mixture received 1 mL of ice-cold TCA and 3 mL of Nash reagent. At 412 nm, the absorbance was measured 22. The formula below is used to compute the hydroxyl radical scavenging activity as a percentage.

HRSA stands for Hydroxyl Radical Scavenging Activity, while Abs control and Abs sample refer to the absorbance of the test solution and control, respectively.
Results and Discussion
Results
A total of five oxazepine derivatives were synthesized and characterized for the retention factor (Rf), solubility, yield (%), melting point (°C) and antioxidant action.
N-benzylidene-2-(4-oxo-2,3-diphenyl-3,4-dihydro-1,3-oxazepin-7(2H)-ylidene)hydrazinecarbothioamide, 8a
Yield – 77%; color – white; melting point – 181-183°C; Rf (n-hexane: ethyl acetate (9:1) – 0.69; soluble in DMSO, slightly soluble in methanol;
IR (cm-1) – 1601.95 (C=S/C=O str), 3054.41 (C-H str), 1311.656 (C-N str), 3435.37 (N-H str), 1525.76 (C=C bend); 1HNMR (δ, ppm) – 6.6 (N-H), 6.8-7.9 (Ar C-H), 8.1 (HC=N); m/z (calculated) – 440.52
N-(4-chlorobenzylidene)-2-(4-oxo-2,3-diphenyl-3,4-dihydro-1,3-oxazepin-7(2H)-ylidene)hydrazinecarbothioamide, 8b
Yield – 74; color – pale yellow; melting point – 170-172°C; Rf (n-hexane: ethyl acetate (9:1) – 0.72; soluble in DMSO, slightly soluble in methanol;
IR (cm-1) – 1605.41 (C=S/C=O str), 3055.14 (C-H str), 1311.656 (C-N str), 3439.11 (N-H str), 1527.23 (C=C bend); 1HNMR (δ, ppm) – 6.7 (N-H), 6.7-7.9 (Ar C-H), 8.2 (HC=N); m/z (calculated) – 474.96
N-(4-hydroxybenzylidene)-2-(4-oxo-2,3-diphenyl-3,4-dihydro-1,3-oxazepin-7(2H)-ylidene)hydrazinecarbothioamide, 8c
Yield – 76; color – yellow; melting point – 151-153°C; Rf (n-hexane: ethyl acetate (9:1) – 0.78; soluble in DMSO, slightly soluble in methanol;
IR (cm-1) – 1609.07 (C=S/C=O str), 3051.68 (C-H str), 1314.65 (C-N str), 3451.24 (N-H str), 1528.19 (C=C bend); 1HNMR (δ, ppm) – 6.7 (N-H), 6.7-7.9 (Ar C-H), 8.1 (HC=N), 5.1 (O-H); m/z (calculated) – 456.52
N-(2-nitrobenzylidene)-2-(4-oxo-2,3-diphenyl-3,4-dihydro-1,3-oxazepin-7(2H)-ylidene)hydrazinecarbothioamide, 8d
Yield – 69; color – brown; melting point – 166-169°C; Rf (n-hexane: ethyl acetate (9:1) – 0.64; soluble in DMSO, slightly soluble in methanol;
IR (cm-1) – 1600.95 (C=S/C=O str), 3053.41 (C-H str), 1310.65 (C-N str), 3435.37 (N-H str), 1524.76 (C=C bend); 1HNMR (δ, ppm) – 6.2 (N-H), 6.8-7.6 (Ar C-H), 8.4 (HC=N); m/z (calculated) – 485.51
N-(3-nitrobenzylidene)-2-(4-oxo-2,3-diphenyl-3,4-dihydro-1,3-oxazepin-7(2H)-ylidene)hydrazinecarbothioamide, 8e
Yield – 71; color – brown; melting point – 165-167°C; Rf (n-hexane: ethyl acetate (9:1) – 0.63; soluble in DMSO, slightly soluble in methanol;
IR (cm-1) – 1601.95 (C=S/C=O str), 3054.41 (C-H str), 1311.656 (C-N str), 3436.37 (N-H str), 1525.76 (C=C bend); 1HNMR (δ, ppm) – 6.2 (N-H), 6.8-7.6 (Ar C-H), 8.4 (HC=N); m/z (calculated) – 485.51
Antioxidant action
The antioxidant action of the synthesized Schiff bases of oxazepine was assessed using DPPH scavenging assay and hydroxy radical scavenging assay.
DPPH scavenging assay
The absorbance of control as well as various concentration of the test solution was measured at 517 nm using UV-visible spectrophotometer and the % DPPH inhibition was measured (Table 1, Figure 2).
Table 1: % DPPH scavenging action of Schiff bases of oxazepine
|
Concentration |
8a |
8b |
8c |
8d |
8e |
|
10 |
13.9566 ± 0.5844 |
25.8116 ± 0.4347 |
35.8303 ± 0.6221 |
4.2816 ± 0.5730 |
6.5761 ± 0.5649 |
|
20 |
19.387 ± 0.4572 |
29.5971 ± 0.5866 |
41.4903 ± 0.4919 |
7.7234 ± 0.5517 |
9.2147 ± 0.5557 |
|
30 |
25.3526 ± 0.4865 |
33.612 ± 0.7481 |
47.3794 ± 0.4861 |
12.0828 ± 0.5457 |
12.7712 ± 0.5433 |
|
40 |
27.8768 ± 0.3157 |
39.9608 ± 0.3786 |
53.8041 ± 0.3367 |
15.3334 ± 0.4881 |
17.0925 ± 0.5147 |
|
50 |
30.6296 ± 0.5933 |
44.3211 ± 0.0198 |
58.929 ± 0.1516 |
19.6928 ± 0.5650 |
20.8021 ± 0.4045 |
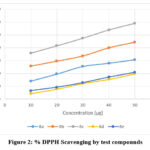 |
Figure 2: % DPPH Scavenging by test compounds |
The IC50 value of each test compound in inhibition DPPH radical was calculated from the graph (Table 2).
Table 2: IC50 of test compounds in DPPH scavenging
|
8a |
8b |
8c |
8d |
8e |
|
|
IC50 |
93.47 µg |
62.37 µg |
34.29 µg |
129.3 µg |
131.04 µg |
Hydroxy radical scavenging assay
The absorbance of control as well as various concentration of the test solution was measured at 412 nm using UV-visible spectrophotometer and the % DPPH inhibition was measured (Table 3, Figure 3).
Table 3: % Hydroxy radical scavenging action of Schiff bases of oxazepine
|
Concentration |
8a |
8b |
8c |
8d |
8e |
|
10 |
7.5777 ± 0.2671 |
18.4333 ± 0.1624 |
28.1245 ± 0.5146 |
2.0402 ± 0.1302 |
3.315 ± 0.0568 |
|
20 |
12.459 ± 0.2458 |
22.0765 ± 0.1694 |
33.6612 ± 0.3188 |
4.153 ± 0.0712 |
6.1202 ± 0.1799 |
|
30 |
17.8508 ± 0.1610 |
25.5008 ± 0.0828 |
38.7132 ± 0.1943 |
8.0697 ± 0.0486 |
9.1603 ± 0.1094 |
|
40 |
20.3641 ± 0.1234 |
31.402 ± 0.4471 |
45.0274 ± 0.0984 |
11.1839 ± 0.0436 |
13.0418 ± 0.0409 |
|
50 |
23.2201 ± 0.2447 |
35.8159 ± 0.2790 |
49.8357 ± 0.1885 |
14.3483 ± 0.1278 |
16.2102 ± 0.0736 |
 |
Figure 3: % HRSA by test compounds. |
The IC50 value of each test compound in inhibition hydroxy radical was calculated from the graph (Table 4).
Table 4: IC50 of test compounds in hydroxy radical scavenging
|
8a |
8b |
8c |
8d |
8e |
|
|
IC50 |
116.00 µg |
82.97 µg |
49.94 µg |
153.12 µg |
152.11 µg |
Discussion
Chemistry
The synthesis of Schiff’s base of oxazepine was achieved in four steps. In the first step condensation reaction occurs between aniline and benzaldehyde leading to the formation of benzylidineaniline with the loss of a water molecule. In the subsequent step, (2+5) cycloaddition occurs between the imine and anhydride leading to the formation of 1,3-oxazepine-4,7-dione 23. Next, the oxazepine dione undergoes a condensation reaction with thiosemicarbazide leading to the formation of thiosemicarbazone derivative which finally undergoes nucleophilic addition by reaction with active carbonyl group of aromatic aldehydes leading to the formation of the desired Schiff’s base compounds. The IR spectra of all the compounds exhibited stretching and bending vibrations due to the presence of functional groups (C=O, C=S, C=N, C-C, C=C, N-H) in all the compounds. The vibrations due to O-H, C-Cl were also found in the corresponding compounds. The proton NMR spectra of the Schiff’s bases depicted chemical shifts due to imine proton, aromatic protons and amine protons.
Antioxidant action
A stable hydrogen or nitrogen-centered free radical called DPPH can accept an electron to transform into a stable diamagnetic molecule. In the presence of suitable reducing agents, DPPH radicals undergo a reaction that results in the loss of colour in a stoichiometric relationship with the amount of consumed electrons, which is quantified spectrophotometrically at 517 nm. It is an indicator of the hydrogen donor capacity of the molecules. The compounds had IC50 in the range of 34.297 µg to 131.04 µg.
The HRS assay is used to determine the scavenging activity of free hydroxyl radicals, such as hydrogen peroxide, which harm body cells, in the presence of various test sample concentrations. The system employed to generate hydroxyl radicals is represented by the ascorbic acid-iron-EDTA model. Ascorbic acid, iron, and EDTA work together in this entirely aqueous environment to produce hydroxyl radicals. The test compounds had IC50 in the range of 49.943 µg to 153.13 µg. The results revealed a dose dependent inhibition of DPPH and hydroxy radicals by the test compounds. Also it was observed that the presence of hydrogen donor group in test compound (8c) was able to inhibit the free radicals better in comparison to compounds containing electron withdrawing substituents (8d, 8e).
Conclusion
Antioxidants are necessary substances that lower or neutralise ROS, shielding organisms from ROS and shielding cells from oxidative damage. The objective of the present investigation is to synthesize 1,3-oxazepine derivatives in a multistep reaction yielding Schiff bases. The Schiff bases would be evaluated for their antioxidant action using in vitro assay (DPPH scavenging assay and hydroxy radical scavenging assay). From the study it could be concluded that the Schiff’s base derived from 1,3-oxazepine-4,7-diones were significant in inhibition of the radicals and are potential candidates for antioxidant action.
Acknowledgement
The authors are thankful to RB Science Research Lab for providing facility for antioxidant study. The authors are also thankful to the management of LNCP, Bhopal for the laboratory facilities for synthesis and SAI Labs, Patiala for the spectral studies.
Conflict of Interest
There is no conflict of interest.
References
- Allamy, A.K.; Mejbel, S.A. World J. Adv. Res. Rev. 2022, 15(1), 662-678
CrossRef - Hanoon, H.D. National J. Chem, 2011, 41, 44-89
CrossRef - Shaimaa, A.; Ahmad, J. M.; Hassan, T. J. Chem. Pharm. Res. 2015, 10, 1000-1011
- Ayad, K.; Israa, B.; Hyder, J. J. Nat. Sci Res. 2015, 15, 69-80
- Nagham, M. J. Sci. Innov. Res 2013, 2, 53-60
- Atyaf, Y.; Nasreen J. Org. Chem. An Indian J., 2016, 12
- Agirbas, H.; Kemal, B.; Budak, F. Med Chem Res, 2011, 20, 1170-1180. https://doi.org/10.1007/s00044-010-9457-4
CrossRef - Serrano-Wu, M. H.; Laurent, D.R.; Chen, Y.; Huang, S.; Lam, K.R.; et al. Bioorganic Med. Chem. Lett. 2002, 12, 2757-2760. https://doi.org/10.1016/S0960-894X(02)00529-2
CrossRef - Abedel-Hahez, A.A.; Abdel-Wahab, B.A. Bioorg. Med. Chem. 2008, 16, 7983-7991. https://doi.org/10.1016/j.bmc.2008.07.064
CrossRef - Hallinan, E.A.; Hagen, T.J.; Tsymbalov, S.; Husa, R.K.; Lee, A.C.; Stapelfeld, A.; Savage, M.A. J. Med. Chem. 1996,39, 609-613. https://doi.org/10.1021/jm950454k
CrossRef - Kubota, K.; Kurebayashi, H.; Miyachi, H.; Tobe, M.,;Onishi, M.; Isobe, Y. Bioorg. Med. Chem, 2011, 19, 3005-3021. https://doi.org/10.1016/j.bmc.2011.03.003
CrossRef - Bajaj, K.; Srivastava, V.K.; Kumar, A. Indian J. Chem. – B Org. Med., 2003, 42, 1149-1155.
- Kurutas, E.B. Nutr. J. 2015, 15: 71.
CrossRef - Liu, Z-Q. Eur. J. Med. Chem. 2019, 189, 112020. doi: 10.1016/j.ejmech.2019.112020
CrossRef - Finkel, T.; Holbrook, N. J. Nature. 2000, 408, 239–247. doi: 10.1038/35041687
CrossRef - Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S. E. Physiol. Rev. 2014, 94, 329–354. doi: 10.1152/physrev.00040.2012
CrossRef - Da Pozzo, E.; De Leo, M.; Faraone, I.; Milella, L.; Cavallini, C.; Piragine, E.; et al. Oxid. Med. Cell. Longev. 2018, 9395804.
CrossRef - Kh. Al-Qatrani, N.H.; Essa, A.H.; Al-Jadaan, S.A.N. Int. J. Sci. Tech. 2019, 14(3), 23-29
- Hassan, M.; Ghaffari, R.; Sardari, S.; Farahani, Y.F.; Mohebbi, S. Res. Pharm. Sci. 2020, 15(3), 281-290
CrossRef - Hussain, Z.; Yousif, E.; Ahmed, A.; Altaie, A. Org. Med. Chem. Lett. 2014, 4, 1
CrossRef - Mishra, N.; Jain, P.; Mishra, B. J. Pharmacol. Biomed., 2017, 1(3), 94-102.
- Ruksar; Chaurey. J. Pharmacol. Biomed. 2021, 5(3), 312-318
- Abid, O.H., Ramadan, A.K. Al-Mustansiriyah J. Sci., 2018, 29(2), 93-100
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









