Optically Important Cu2ZnSnS4 (CZTS) Nanoparticles Synthesis using a Hydrothermal Route with Citric Acid as a Structure Directing Agent
Bhavya Pandey and Y C Goswami*
and Y C Goswami*
ITM University, Jhansi Rd, Turari, Gwalior, Lakhnotikhurd, Madhya Pradesh 474001, India
Corresponding Author E-mail: y_goswami@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/390429
Article Received on : 01 May 2023
Article Accepted on : 10 Aug 2023
Article Published : 24 Aug 2023
Reviewed by: Dr. Hamid reza Ghorbani
Second Review by: Dr. M. Balachander
Final Approval by: Dr. Gunawan
Copper zinc tin sulphide (CZTS) has emerged as a highly promising, cost-effective, and environmentally friendly material for solar energy conversion via photovoltaic and photocatalysis. This paper presents the synthesis of quasi Cu2ZnSnS4 nanoparticles using a hydrothermal route with citric acid as a structure-directing agent. The CZTS nanoparticles' morphological, structural, and optical properties were comprehensively analysed using X-ray diffraction, scanning electron microscopy, and UV-Vis absorption studies. The results demonstrate the successful synthesis of CZTS nanoparticles with a novel narrow size distribution, making them excellent candidates as absorber layers in solar cells. This discovery holds significant potential for advancing low-cost and efficient solar energy conversion technologies. By harnessing the unique properties of CZTS, this research offers a promising solution towards sustainable energy production and a greener future.
KEYWORDS:Hydrothermal route; Optically important Cu2ZnSnS4 (CZTS); Nanoparticles synthesis
Download this article as:| Copy the following to cite this article: Pandey B, Goswami Y. C. Optically Important Cu2ZnSnS4 (CZTS) Nanoparticles Synthesis using a Hydrothermal Route with Citric Acid as a Structure Directing Agent. Orient J Chem 2023;39(4). |
| Copy the following to cite this URL: Pandey B, Goswami Y. C. Optically Important Cu2ZnSnS4 (CZTS) Nanoparticles Synthesis using a Hydrothermal Route with Citric Acid as a Structure Directing Agent. Orient J Chem 2023;39(4). Available from: https://bit.ly/45iC940 |
Introduction
Metal nanoparticles and composites gained significant attention due to their remarkable properties and diverse applications 1-6. Cu and Ag nanoparticles exhibit unique optical, electrical, and catalytic characteristics, making them promising candidates for various fields, including electronics, photonics, biomedicine, and environmental remediation 7-11. Their large surface area-to-volume ratio and tunable properties at the nanoscale provide exciting opportunities for cutting-edge research and technological advancements. Among other, quaternary composites, Copper zinc tin sulphide (CZTS) has gained popularity as a sustainable alternative to conventional photovoltaic materials for solar energy conversion, owing to its non-toxic and earth-abundant nature. CZTS devices have demonstrated high efficiency for different solar cell applications 12. With the increasing global attention to environmental crises, semiconductor thin film materials-based second-generation solar cells have become significant. With an optical band gap of 1.5 eV and an absorption coefficient of 104 cm-1, CZTS is categorised as a quaternary semiconductor, which is advantageous for solar cell applications 14-15. Utilising a solution deposition method based on hydrazine has led to a mixed sulfo-selenide device achieving an efficiency of 11.1%., while sputtered pure sulfide CZTS devices have achieved up to 8.4% efficiency16-17. CZTS, a Kesterite, has replaced commercially available CIGS solar cells due to its optoelectrical properties, stability, and cost-effectiveness 18. The band structure of CZTS is similar to other Cu-chalcogenides, incorporating Se anions resulting in a higher VBM and lower band gap 19.
Several methods have been reported for synthesising CZTS, each with advantages and limitations. Spray pyrolysis technique is widely used for CZTS synthesis, involving the atomisation of a precursor solution followed by pyrolysis on a substrate. This method boasts exceptional control over film thickness, composition, and morphology, making it highly suitable for device fabrication 20. As highlighted by Wang et al., the spray pyrolysis technique enables the deposition of CZTS films with superior quality, characterised by uniform composition and controlled thickness, thus holding great promise for large-scale CZTS solar cell production. However, certain concerns associated with this method must be addressed. Firstly, the high-temperature processing involved may pose challenges in terms of energy consumption and overall sustainability. Secondly, using toxic precursors raises environmental and health-related issues, further hindering the method’s long-term viability. Therefore, efforts should be directed towards developing alternative and greener approaches to ensure the sustainable production of CZTS solar cells.
Another approach for CZTS synthesis is the solution-based method, which involves the chemical reaction of precursors in a solution. This method offers simplicity, scalability, and the possibility of large-scale production 21. A solution-based technique is a cost-effective and environmentally conscious means of synthesising CZTS, making it a viable option for producing CZTS thin films on a large scale. However, the control over composition and morphology is relatively lower than the solvothermal method, which may affect the overall performance of CZTS films.
One of the widely used methods for CZTS synthesis is the solvothermal method, which involves the reaction of precursors in a solvent at high temperature and pressure. This method offers precise control over the composition and morphology of CZTS nanoparticles, resulting in high-quality films with excellent optoelectronic properties 22. Zhou et al. noted that the solvothermal method allows for the facile synthesis of CZTS nanoparticles with tunable bandgaps, making it a promising approach for CZTS-based solar cells. However, the high energy consumption and use of toxic solvents are significant concerns associated with this method, limiting its scalability and sustainability.
In recent years, there has been growing interest in developing environmentally friendly methods for CZTS synthesis. For instance, the hydrothermal method, which uses water as a solvent, has been explored as a sustainable approach for CZTS synthesis. Li et al. emphasized that the hydrothermal method is a green synthesis route for CZTS, offering a sustainable and low-cost approach for producing CZTS thin films 23. Meanwhile, environmentally friendly methods such as hydrothermal synthesis and the use of renewable precursors are reported 24. Water is much cheap, readily available and has no toxic effect on environment. So, if we use water, the hydrothermal route will be developed and water will replace the organic solvent, which is more favorable for synthesising CZTS Nano crystalline materials 25,26.
In 2014, Samrat Sarkar and colleagues 27 presented their findings on synthesising 3D-hierarchical microspheres of Cu2ZnSnS4 (CZTS) through a self-sacrificial template directed hydrothermal method, with the addition of citric acid. They optimised the phase purity and morphology of the final product. The addition of citric acid was found to reduce the possibility of phase segregation while also influencing the crystal growth process to facilitate the formation of the desired 3D structure.
In the same year, Xiuquan Gu and co-authors 28 reported on the synthesis of CZTS nanoparticles via a one-step solvothermal approach. The synthesised nanoparticles had a predominantly single kieserite structure, with a fundamental band gap of 1.54 eV. The researchers observed improved performance, which they attributed to the more excellent stability, sensitivity to sunlight, and resulting photo electrocatalytic activity of the CZTS electrodes.
Yongtao Qu and co-authors 29 reported in their study that the synthesis of Cu2ZnSnS4 (CZTS) nanoparticle inks through the injection of metal precursors into a hot surfactant represents an appealing approach for fabricating Earth-abundant thin-film photovoltaic absorber layers of Cu2ZnSn(S, Se)4 (CZTSSe). The researchers discovered that the reaction time, temperature, and cooling rate of the concentration of acceptor levels in CZTSSe photovoltaic absorbers and led to the nanoparticle fabrication process impacted the doping level, secondary phases, and crystal structure of the final product. In particular, the extension of the reaction duration provided a novel method to enhance increased device efficiency.
Materials synthesis often requires the use of structure-directing agents (SDAs) to manage the dimensions, form, and characteristics of the resulting substances. control the size, shape, and properties of the resulting materials. In recent years, citric acid (CA) has emerged as a versatile and environmentally friendly SDA in synthesising various materials due to its unique properties and advantages 30.
Citric acid (CA), a natural organic acid found in fruits, has carboxyl and hydroxyl groups that can coordinate with metal ions, making it an effective SDA in developing metal-organic frameworks (MOFs) and other coordination polymers. For instance, CA has been used as an SDA in the synthesis of MOFs with diverse topologies and properties, including UiO-66, MIL-53, and ZIFs (zeolitic imidazolate frameworks) (Furukawa et al., 2010; Tranchemontagne et al., 2008) 31,32. The use of citric acid as a structure-directing agent allows for the facile synthesis of MOFs with tunable porosity and properties, making it a versatile approach for materials design
One of the unique features of Citric acid is its ability to act as both a chelating agent and a reducing agent, making it suitable for synthesising metal nanoparticles. CA can effectively reduce metal ions and stabilize the resulting nanoparticles due to its multiple functional groups, such as carboxyl and hydroxyl groups, which can interact with metal ions (Makarov et al., 2014) 33. For example, CA has been utilised as an SDA in the synthesis of metal nanoparticles with controlled size, shape, and surface properties (Skrabalak et al., 2007; Tripathi et al., 2018) 34,35. This studies shows that the use of citric acid as a structure directing agent allows for the synthesis of metal nanoparticles with precise control over size, shape, and properties, making it a versatile approach for nanoparticle synthesis.
Furthermore, CA has been employed as an SDA in the synthesis of other materials, such as metal oxides, metal phosphates, and hybrid organic-inorganic materials. For instance, CA has been used in the synthesis of mesoporous metal oxides, including TiO2, ZrO2, and Fe2O3, with high surface area and controlled porosity for various applications, such as catalysis and energy storage 36,37. CA has also been used as an SDA in synthesising organic-inorganic hybrid materials, such as hybrid perovskites, by coordinating with metal ions and stabilising the resulting materials 38,39.
The use of citric acid as a structure-directing agent offers several advantages. First, CA is a natural and biocompatible acid, making it environmentally friendly and suitable for various applications. Second, CA has multiple functional groups that can coordinate with metal ions and reduce them, providing versatility in materials synthesis. Third, CA allows for the precise control of size, shape, and properties of the resulting materials, enabling tailored materials design. The use of citric acid as a structure directing agent offers a sustainable and versatile approach for materials synthesis, with potential applications in diverse fields including catalysis, energy storage 39.
In the present work we reported a new hydrothermal method for synthesising kesterite-type CZTS microspheres with citric acid as the structure-directing agent. Our findings reveal that citric acid plays a crucial role in promoting the formation of phase-pure CZTS.
Experimental
Materials
The materials used for the synthesis were AR grade Cupric sulphate extra pure (CuSO4.5H2O), Zinc(II)Sulphate Heptahydrate (ZnSO4 .7H2O), Thiourea (Thiocarbamide) CH4N2S, Stannous Chloride (SnCl2.2H2O), and 0.02 mol of Citric Acid (0.192gm) purchased from CDH and Ranbaxy. All of these materials were of high purity and were carefully weighed and measured to ensure accurate results. During the synthesis of CZTS microspheres, the chemicals were utilised without further purification. The synthesis process was conducted under controlled conditions to ensure the materials were not contaminated or degraded.
Synthesis
Step 1: Development of Precursor
A typical synthesis involved dissolving 0.1 mol of Copper Sulphate (approximately 1.248gm), 0.05 mol of Zinc Sulphate (0.718gm), 0.05 mol of Tin(IV) Chloride (0.564gm), and 0.6 mol of Thiourea (2.283gm) in 250 ml of distilled water. The solution was stirred using a magnetic stirrer until a white cloudy solution was obtained. Then, 0.02 mol of Citric Acid (0.192gm) was added to the solution (Sample 1).
Step 2: Hydrothermal route
Next, the mixture was transferred into a Teflon-lined stainless steel autoclave with a capacity of 500 ml. The autoclave was then heated to 180 °C using a hot air oven for 24 hours. After heating, the autoclave was allowed to cool down naturally to room temperature. The resultant black sample was then washed several times with ethanol and distilled water alternately to eliminate any by-products.
The final product was then vacuum dried at 60 °C for 4 hours before being characterised.To investigate the impact of citric acid on the synthesis process, the procedure was repeated by adding various amounts of citric acid to the solution of metal salts while keeping the temperature, time, and concentration constant. Sample B utilized 0.04 M citric acid, Sample C utilised 0.08 M citric acid, and Sample D utilized 0.12 M citric acid.
The collected samples underwent various analyses, including structural studies using X-ray diffractograms, morphological studies using SEM, and optical studies using UV-Visible and PL spectroscopy. To obtain the X-ray diffractograms, the samples were analysed using an X Pert Pro PAN analytical X-ray diffractometer within the 2θ range of 20 to 800C using CuKα radiation of wavelength 1.546 Å.
SEM micrographs were obtained using a TECHAI G2 F20 operating at 300 kV. Additionally, UV-Vis spectra were obtained using a Perkin Elmer alpha 2 within a wavelength range of 200nm-800nm.
Results and Discussion
Structural studies: XRD analysis.
The X-ray diffraction (XRD) pattern presented in Figure 1 shows all the identifiable peaks for the sample. The kesterite structure of the CZTS is evident, with the (112), (220), and (312) phases showing distinct peaks 40. These planes were observed in all samples, confirming the formation of the kesterite CZTS phase. Additionally, a preferred (112) orientation was observed, consistent with previous reports on CZTS. Copper peaks were also detected at 2θ = 33.88.
The Debye Scherrer formula was used to determine the size of the crystalline particles 41 in different samples. The formula considers the diameter of the particles, the Bragg angle, the width of the dominant peak, and the wavelength of the X-ray used. Results indicated that the grain size increased as the quantity of citric acid used in the growth of the samples varied. It is observed that with the addition of citric acid, the impurities in the model are reduced. As the citric acid increases gradually and slowly, the material becomes a monophase material.
 |
Figure 1: X-ray diffractograms of CZTS particles (Sample 1-4). |
Morphological Studies
By examining the FESEM micrographs, it is possible to gain significant knowledge about the morphology of CZTS particles produced using various citric acid concentrations. It was observed that when the samples were grown with lower citric acid concentrations, the CZTS particles exhibited irregular shapes and sizes. However, when the concentration of citric acid was increased to one-quarter or half of the total metal ion concentration, the morphology of the CZTS particles changed drastically, resulting in the formation of spherical superstructures that resembled flowers, with diameters exceeding 4μm. Furthermore, the FESEM analysis also revealed the chain-like structures of small spherical nanostructures connected to the larger microspheres. The flower-like morphology of the spheres was mainly attributed to several interconnected nanosheets stacked together on the surface of the microspheres. This unique morphology is expected to have a significant impact on the optical and electrical properties of the CZTS particles, making them suitable for various photovoltaic applications.
Interestingly, when the citric acid concentration was increased further, the microspheres’ size remained unchanged, but the morphology of the CZTS particles slightly altered. The FESEM analysis suggest an increase in particle size when the samples were grown with various quantities of citric acid. This observation could be attributed to the effect of citric acid on the growth kinetics of the CZTS particles, leading to the formation of larger particles with different morphologies. Overall, the FESEM analysis provides valuable information on the morphology of the CZTS particles grown with different citric acid concentrations 42. These insights could be helpful in developing novel synthesis strategies to control the morphology and size of CZTS particles for various applications, including solar cells, sensors, and catalysis. Citric acid plays a crucial role in reducing the likelihood of phase segregation and facilitating the formation of the desired structure by affecting the crystal growth process.
 |
Figure 2: SEM micrographs for CZTS particles (Sample 1-4). |
Optical Studies
In addition to the FESEM analysis, optical studies were carried out to investigate the impact of citric acid concentration on the energy bandgap of the CZTS particles. UV spectrophotometer was used to obtain absorption spectra of the samples synthesized under various citric acid concentrations. Tauc plots 43 were then constructed using the graphs of (ahv)2 versus hv, and the curved extrapolation provided the band gap values of the samples.
The results showed an increase in the energy bandgap of the CZTS particles from 1.50 eV to 1.55 eV with increasing citric acid concentration. This finding was consistent with the trend observed in Table 1, which showed that the energy bandgap of the CZTS material varied in the range of 1.4 to 1.5 eV. Moreover, it was observed that the energy bandgap increased slowly as the citric acid concentration was increased. The change in energy bandgap has significant implications for the optical and electrical properties of the CZTS particles. A larger bandgap means that the CZTS particles have a higher potential to absorb higher energy photons and convert them into electricity. Additionally, the increase in bandgap could improve the stability and durability of the CZTS particles, making them more suitable for photovoltaic applications.
Overall, the optical studies provided valuable insights into the relationship between citric acid concentration and the energy bandgap of the CZTS particles. The increase in bandgap with increasing citric acid concentration suggests that citric acid plays a critical role in modulating the optical properties of the CZTS particles, which could be helpful in designing new and efficient synthesis strategies for these materials.
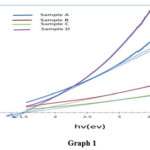 |
Graph 1 Click here to View Graph |
Table 1: Calculated Band gap for Samples
|
Sample |
Energy bandgap (Eg) |
|
A or 1 |
1.50ev |
|
B or 2 |
1.51ev |
|
C or 3 |
1.55ev |
|
D or 4 |
1.52ev |
Conclusions
Synthesis and characterisation of CZTS particles obtained with citric acid have provided valuable insights into the properties of these materials. The use of citric acid as a complexing agent has been found to play a critical role in modulating the morphology and energy bandgap of the CZTS particles. Specifically, it was observed that increasing the citric acid concentration led to the formation of flower-like spherical superstructures composed of interconnected nanosheets, as well as an increase in the energy bandgap from 1.50 eV to 1.55 eV.
The FESEM and UV
spectrophotometer analyses have demonstrated that
The concentration of citric acid has a notable effect on the characteristics of
the CZTS particles.. These findings could be useful in designing new and
efficient synthesis strategies for CZTS particles with tailored properties for
photovoltaic applications. Additionally, the CZTS particles obtained with
citric acid have the potential for improved stability and durability, which are
important considerations for their practical use.
Overall, the study has highlighted the importance of the careful selection of complexing agents in synthesising CZTS particles and the need for a comprehensive characterisation approach to understanding their properties fully. The results of this study could inform the development of new materials with improved performance for renewable energy applications.
Acknowledgements
The Authors are thankful to the P C Ray Centre ITM University Gwalior and IIT Mandi for providing characterisation facilities.
Conflict of Interest
The authors have no conflicts of interest to declare.
Reference
- Ghorbani, H. R; Fazeli ,I; Fallahi, A. A. Orient J Chem 2015, 31(1), 515-517.
CrossRef - Ghorbani, H. R.;. Orient J Chem 2014;30(2). 803-806.
CrossRef - Ghorbani, H.R.; Attar, H.; Safekordi, A. A; and Sorkhabadi, S.M. R. Asian J. Chem. 2011, 23(11), 5111-5118
- Ghorbani, HR. IET Nanobiotechnology. 2014, 8(4), 263-6.
CrossRef - Kaundal, J. B; Goswami, Y. C; Sharma, R. Orient J Chem 2022, 38(3), 766-770.
CrossRef - Lakshminarayanan, V.; Bhattacharya, I.; Advances in Optical Science and Engineering, Springer Proceedings in Phy. 2014,166, 533-539.
- Ghorbani, H.R.; and Molaei, M. Mater. Res. Express 2017, 4(6) 065017.
CrossRef - Ghorbani, H. Chemical Engineering Communications, 2014, 202:11, 1463-1467.
CrossRef - Khodashenas B.; Ghorbani , H.R. IET Nanobiotechnol. 2016;10(3):158-61.
CrossRef - Nagaich, S.; Goswami, Y. C. Fifth International Conference on Advanced Computing & Communication Technologies 2015, 1, 165-168.
- Goswami, Y.C.; Kaundal, J.B.; Begzaad, S. et al. Photocatalytic degradation of Methyl Red dye using highly efficient ZnO/CdS hierarchical heterostructures under white LED. J IRAN CHEM SOC 2023, 20, 1681–1697.
CrossRef - Ravindiran, M.; & Praveenkumar, C.; Renewable and Sustainable Energy Reviews, 2018, 94, 317–329.
CrossRef - Song, X., Ji, X., Li, M., Lin, W., Luo, X., & Zhang, H. (2014). International Journal of Photoenergy, 2014, 2014, 1–11.
CrossRef - Mahajan, S.; Stathatos, E.; Huse, N.; Birajdar, R.; Kalarakis, A.; & Sharma, R. Materials Lett. 2018, 210, 92–96.
CrossRef - Lee, S. G.; Kim, J.; Woo, H. S.; Jo, Y.; Inamdar, A. I.; Pawar, S. M. Current Appl. Phy. 2014 14(3), 254–258.
CrossRef - B. Shin, B; O. Gunawan, O; Zhu, Y.; Bojarczuk, N.A. S.; Chey, S.J.; Guha, S. Prog. Photovolt: Res. Appl. 21, 2013, 72–76.
CrossRef - Nazligul, A.S.; Wang, M.; Choy, K.L. Sustainability 2020, 12, 12(12), 5138.
CrossRef - Chen, S.; Walsh, A.; Yang, J.-H.; Gong, X.G.; Sun, L.; Yang, P.-X.; Chu, J.-H.; Wei, S.-H. Phys. Rev. B 2011, 83, 125201.
CrossRef - Zhou, Y.; Wang, C.; Yang, M.; Yin, X.; & Liu, W. (2018). Journal of Materials Chemistry A, 2018 6(18), 8335-8350.
- Xu, Y.;Yang, L., Huang, X., Zhao, X., & Zhang, Z. (2019). Materials Science in Semiconductor Processing, 2019, 100, 104594.
- Wang, X.; Li, B.; & Wu, H. Journal of Materials Science & Technology, 2017 33(10), 1123-1134.
- Li, S.; Li, G.; & Fan, J. Journal of Alloys and Compounds, 2020, 824, 153861.
CrossRef - Dai, X.; Chen, S.; Fan, X.; & Wu, L. Journal of Materials Chemistry C, 2018 6(11), 2797-2806.
- Xia, Y.; Chen, Z.; Zhang, Z.; Fang, X.; & Liang, G. (2014). Nanoscale Research Lett. 2014, 9(1), 208.
CrossRef - Shinde, S.S. Journal of Semiconductors 2015 36(7) 073003.
CrossRef - Sarkar, S.; , Bhattacharjee K.; Das, G.C. and Chattopadhyay, K.K. Cryst Eng Comm 2013 16(13), 2634-2644.
CrossRef - Xiuquan; G.; Zhang S.; Qiang Q.; Zhao Y.; and Zhu L., ACS Appl. Mater Interfaces 2014 43(7), 2709–2714. (2014)
CrossRef - Qu, Y., Zoppi, G., and Beattie, N. S. Prog. Photovolt: Res. Appl. 2016 24: 836– 845.
CrossRef - Sweta L.; Heera K. & Milind U. GSC Biological and Pharmaceutical Sciences. 2021 17. 085-093.
CrossRef - Furukawa, H.; Kim, J.; Ockwig, N. W.; O’Keeffe, M.; & Yaghi, O. M. Journal of the American Chemical Society 2010, 132(26), 9508-9510.
- Tranchemontagne, D. J.; Hunt, J. R.; & Yaghi, O. M. Tetrahedron, 2008 64, 8553-8557.
CrossRef - Makarov, V. V.; Love, A. J.; Sinitsyna, O. V.; Makarova, S. S.; Kaminsky, I. V.; Taliansky, M. E.; & Kalinina, N. O. Acta Naturae, 2014, 6(1), 35-44.
CrossRef - Skrabalak, S. E.; Chen, J.; Sun, Y.; Lu, X.; Au, L.; Cobley, C. M., & Xia, Y. Accounts of Chemical Research, 2007, 41(12), 1587-1595.
CrossRef - Tripathi, K. M.; Ramasamy, K.; & Pugazhenthiran, N. Materials Chemistry and Physics 2018, 210, 305-315.
- Mai, H. X.; Zhang, Y. W.; Sun, L. D.; Yan, C. H. (2012). Journal of the American Chemical Society, 2012 134(49), 20419-20432.
- Deng, D.; Chen, X.; Yu, L.; Wu, L. M.; Liu, H.; Wang, X.; & Zhou, Y. Journal of Materials Chemistry A, 2013 1(21), 6402-6410.
- Feng, L.; Li, X.; Li, J.; Li, L.; & Li, G. Materials Lett. 2018, 215, 21-24.
CrossRef - Kim, H. S.; Jeong, M.; Hoh, H. Y.; Lee, J. Y.; Kim, S. S.; & Seok, S. I. Journal of Materials Chemistry, 2011, 21(28), 10234-10237.
- Pinzón, D.L.S.; Cuaspud, J.A.G.; E. Vera López, E.V.; Schmal, M. Materials Research. 2021; 24(3): e20200290.
CrossRef - N Kumar, LP Purohit, YC Goswami, Spin coating of ZnS nanostructures on filter paper and their characterisation, Physica E: Low-dimensional Systems and Nanostructures 2016 83, 333-338.
CrossRef - Pal, D; Singh, G.; Goswami, Y.C.; Kumar V. Journal of Materials Science: Materials in Electronics 2019, 30, 15700-15704.
CrossRef - Goswami, Y.C. Kansal A., AIP Conference Proc. 2009, 1147 (1), 223-228

This work is licensed under a Creative Commons Attribution 4.0 International License.









