Exploration of Bioplastics: A Review
Ritu Saharan* and Jyoteshna Kharb
Department of Chemistry, University of Rajasthan, Jaipur-302004, India.
Corresponding Author E-mail: ritu.saharan84@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380403
Article Received on : 05 Jul 2022
Article Accepted on : 10 Aug 2022
Article Published : 16 Aug 2022
Reviewed by: Dr. Aditya Reddy
Second Review by: Dr. Vijay L. Gurav
Final Approval by: Dr. Tawkir Sheikh
The marvellous and versatile properties of synthetic plastics make them an indispensable part of human lives. But in the recent years, plastic pollution has become the biggest environmental concern for the whole world globally. Environmental distress over plastic pollution associated with a rising debate over fossil fuel dependence and abatement have brought the attention of researchers towards finding a suitable alternative to plastics i.e., bioplastic. Bioplastics are specially designed to have lower carbon footprint, less dependent on natural resources, energy efficiency, environmental safety and sustainability. These are bio-resources based polymers which have the potential of substituting conventional petroleum-based plastics. This review article summarizes need for developing eco-friendly alternative to plastics, bioplastics, importance of bioplastic, advantages of bioplastics over plastics and current trends in production of bioplastics. It also highlights types of bioplastics based on various sources and a variety of bioplastic materials such as starch, cellulose, chitosan, chitin, polyhydroxyalkanoates, polylactic acid, Bio-PE, Bio-PET, Bio-PBS, etc., their synthesis, applications and biodegradability. A comparative analysis of both natural and bio-based polymers in term of their availability, nature, structure, properties such as thermal stability, biodegradability, tensile strength, etc. has also been highlighted.
KEYWORDS:Bioplastics; Eco-safety; Energy efficiency; Natural resources; Sustainability
Download this article as:| Copy the following to cite this article: Saharan R, Kharb J. Exploration of Bioplastics: A Review. Orient J Chem 2022;38(4). |
| Copy the following to cite this URL: Saharan R, Kharb J. Exploration of Bioplastics: A Review. Orient J Chem 2022;38(4). Available from: https://bit.ly/3KAKqqt |
Introduction
“Plastics” as synthetic polymers were proposed almost hundred years ago and nowadays these are the highest produced, used, widely available and most dynamic materials 1-3. Plastics find a significant role in almost every place in our daily life ranging from food packaging, pharmaceutical and communication technology to technical applications such as electronics and automobiles 4-11. Plastics are very useful as synthetic polymer due to various properties. The structure of plastics can be chemically altered to various shapes and strengths to produce higher molecular weight, less reactive, highly durable and non-biodegradable products 12-17. Due to their versatile properties, plastic production has increased substantially in 2020 by almost 36% since 2010 18.
Need for developing eco-friendly alternative to plastics
Even though plastics have a lot of advantages but certain disadvantages also. Most of plastics are derived from fossil resources and these are non-renewable and limited in nature. Due to excessive production of plastic and its products, these natural resources are depleting at a very fast rate. Apart from those plastics decay very slowly which can cause the plastic products to persist in the landfills for hundreds or even thousands of years which led to environmental pollution 19-29. Plastic waste has become a global challenge 30. Nearly half of the polymers developed by man end in one-way or short-lived products, which mostly accumulates in solid waste landfills, fresh water bodies and in the oceans, where they get degraded into ‘microplastics’ over time and prove harmful to marine organisms and lastly end up on our plates 31-35. Plastics remain suspended on the surface of almost every ocean of the world. One of the notable example of this plastic accumulation zone is The Great Pacific Garbage Patch that is located between Hawaii and California. Almost 94 79,000 tonnes of plastic waste is piled up in this area of 1.6 km2. About 8% of this plastic waste is present in the form of microplastics and the remaining is present as floating form of plastic 36.
Synthetic plastic such as polystyrene, polyethylene, poly vinyl chloride, etc. derived from non-renewable petrochemical resources release greenhouse gases such methane, carbon monoxide, nitric oxide, etc. which eventually has an immense effect on increasing global warming 37,38. On a global trend, both production of plastic from petro-based sources and incineration of plastic waste account to about 400 million tonnes of carbon dioxide every year 39. Burning of these plastic products also emits toxic substances which prove to be hazardous to the environment and also to the human beings as these toxins cause respiratory diseases and many more health issues 40.
Seeing all the issues, we can say that plastic pollution affects environment at all levels right from its synthesis to discarding and incineration 41-44. No doubt, plastic products contribute largely in the development nowadays, but this development is possible only when we concern about the wellbeing of the environment. And that is the reason, there is augmenting interest in uncovering the materials which have alike properties as those of petro-based plastics 45.
Further, degrading environment, depletion of natural resources, rising prices of fossil fuels and problem of waste disposal have also paved the way of research towards eco-friendly alternative to plastics 46-59. This eco-friendly alternative is Bioplastic 60,61.
The synthesis and use of bioplastics is considered as more sustainable activity in comparison to production of plastics which further opens up opportunities to overcome depletion of natural resources and environmental hazards caused by plastics 62-72.
Bioplastics
According to European Bioplastics, a plastic material is said to be bioplastic if it is either bio-based or biodegradable or possess both the properties 73. International Union of Pure and Applied Chemistry (IUPAC) has defined bioplastic in the following way, “a bioplastic is derived from biomass or monomers derived from biomass and which, at some stage in its processing into finished products, can be shaped by flow” 74.
Bioplastics are derived from organic materials found in nature such as polysaccharides, lipids and proteins. Different polysaccharides used for synthesis of bioplastics are cellulose, starch, lignin, chitin, dextrin, pectin, gum, etc. Casein, gelatin and gluten, and animal fats and plant oils are the naturally occurring proteins and lipids respectively form which bioplastic is obtained 75-81.
Latest advancements are evolving to expand bioplastic production from plant based renewable waste substances, biomass, microbial and microalgal cells so that there is no competition with agricultural and food resources. These sources of bioplastic are obtained mainly from organic wastes generated from food waste, corn and sugarcane waste, vegetable waste, agricultural waste, household kitchen waste, by-products of wood industries, etc. 82.
Utility of greenhouse gases such as carbon dioxide for synthesis of bioplastic is also one of the most sustainable carbon upcycling perspectives that is attaining an enormous recognition now-a-day 83. It will eventually promote minimal carbon footprint for the production of bioplastics84.
Now-a-days researchers and scientists also focus on designing novel bio-composites which are comprised only of bio-based materials having preferred functionalities and properties that are apt for various technological applications 85-87. Blending of different bio-based polymers is also in trend to enhance their versatility and efficiency in terms of biodegradability and recyclability 88.
Advancements in industrial biotechnology propose numerous synthetic routes such as bio-catalytic transformation and chemo-enzymatic catalysis for developing varied monomers and polymers which are bio-based and derived from biomass and renewable feedstocks 89-91.
The biodegradable plastics disintegrate into water, carbon dioxide, humus, biomass, and different other natural substances which are not harmful for the environment.
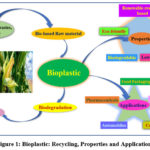 |
Figure 1: Bioplastic: Recycling, Properties and Applications. |
Importance of bioplastics
These bioplastics comprising of both naturally and chemically derived substances from natural and renewable resources are being planned to exhibit 46,76,92,93:
Complete biodegradability,
Decrease toxicity,
Lower carbon footprint,
High recyclability,
Sustainability and
Environment-friendliness
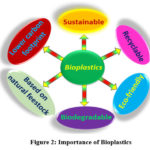 |
Figure 2: Importance of Bioplastics. |
These bioplastics have replaced petro-based conventional plastics and are extensively used for various industrial and environmental applications 94.
Advantages of Bioplastics over plastics 95-98
The bioplastics show various kinds of advantages over the conventional petroleum-based plastics.
Table 1: Advantages of Bioplastics over Plastics
|
|
Bioplastics |
Conventional Plastics |
|
Sources |
Partly based on renewable sources (natural feedstock) |
Based on non-renewable sources (petrochemical sources) |
|
Carbon Footprint |
Comparatively lower |
Much higher |
|
Energy Efficiency |
Production requires less energy |
Production requires more energy |
|
Eco-safety |
More environmental-friendly |
Causes environmental pollution |
|
Biodegradability |
Either completely or partly biodegradable |
Mostly non- biodegradable |
Various terms
Degradation
The process in which a polymer breaks down into smaller particles by the action of certain abiotic factors such as sunlight (UV radiations), air, or through any microbial process. Polyethylenes are the most common type of degradable plastics.
Biodegradation
The process of decomposition of a polymer into tiny fragments through biological activities and carbon dioxide, methane and water are formed as a outcome of biodegradation.
Bio- based plastics
The plastics that are either biodegradable or bio-based i.e. they are derived from naturally available resources or biomass in some way or the other. For example, Bio PE, Bio PVC, etc.
Compostable Plastics
The plastics that undergo biological decomposition in compost sites forming water, biomass, carbon dioxide and other inorganic compounds without leaving any harmful materials in the environment 99.
Conventional Plastics
The plastics that are based on petro-products i.e. derived from fossil fuels 100.
Current Trends in production of Bioplastics
At present, bioplastic materials contribute about one percent to total plastic production but the market is expanding continuously. In 2021, the global production scope of bioplastics was around 2.42 million tonnes, which is estimated to increase to approximately 7.49 million tonnes by 2026.
Around 64% of all the bioplastic materials synthesized are biodegradable which includes PBAT (polybutylene adipate terephthalate), PLA (polylactic acid), PHA (polyhydroxy alkanoates), PBS (polybutylene succinate), starch blends and others. Among them, PBAT contributes approximately 19.2% of the total global production of biodegradable bioplastics. Mainly PLA about (18.9%), starch blends (16.3%), other biodegradable plastics (10%) including PHAs (1.8%) and PBS (3.5%) are synthesized on industrial scale.
Bio-based and non- biodegradable plastics contribute to around 36% to the total global production of bioplastics. Majority of them include bio-based PET (polyethylene terephthalate), bio-based PE (polyethylene), bio-based PA (polyamides) and PEF (polyethylene furanoate), a new biopolymer that is anticipated to enter the market in 2023 101.
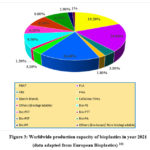 |
Figure 3: Worldwide production capacity of bioplastics in year 2021 (data adapted from European Bioplastics) 101 |
Types of Bioplastics
These biopolymeric materials are distinguished on the basis of their origin; natural and synthetic. Natural bioplastics are formed as a result of continuous evolution in nature while synthetic bioplastics are produced through significant research and progress in this field. Both these processes result in emergence of materials possessing characteristics for varied applications 102.
Natural bioplastics can further be classified into three types based on their sources of production which include production from biomass resources (polysaccharides, polypeptides, lipids), from microorganisms (polyhydroxybutyrate, polyhydroxyalkanoates) and from biotechnological applications (polylactide, poly lactic acid). Synthetic bioplastics can be divided into three categories namely aliphatic polyesters (polycaprolactone), aliphatic copolyesters (polybutylene succinate) and aromatic copolyesters (polybutyrate adipate terphthalate) 103.
Considering the comprehensive properties of both natural and synthetic biopolymers, they both accomplish a pervasive and vital role in every sphere of life. These biodegradable polymers reveal the occurrence of biodegradation by having a period of fruitfulness.
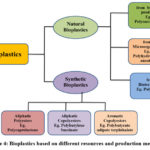 |
Figure 4: Bioplastics based on different resources and production methods. |
Bio-based Natural polymers
Bioplastic derivatives obtained from different natural resources like cellulose, starch, chitin, chitosan, etc. have allured substantial attention of researchers to replace the typical petro-based plastics 104-106. These naturally existing polymers and their blends stand at highest as far as production capacity of bio-based polymers is considered worldwide 107,108.
Starch
Starch is the naturally occurring polysaccharide and amplest biopolymer available on the earth. It is the vital carbohydrate product of plants that is stored for energy. Starch is obtained from a variety of sources such as potatoes, rice, maize, wheat, etc. 109.
It contains a huge number of glucose units associated by 1,4- and 1,6-gycosydic linkages which results in formation of two types of units:
Amylose (linear and helical structures)
Amylopectin (branched structure).
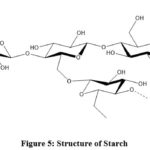 |
Figure 5: Structure of Starch. |
The ratio of amylose/amylopectin has a great impact on the characteristics of starch 110. Due to the significant utility of starch including appreciable availability, renewability, cost effectiveness and biodegradability, it has been looked upon as a promising substitute material for synthesizing bio-based polymers 111-118.
There are strong intermolecular and intramolecular hydrogen bonds among starch polymers which makes it highly crystalline in nature. Due to this, it has a very high melting temperature which is near to degradation temperature and this hinders the way of melting during synthesis of starch films. Hence starch is modified using physical and chemical processes during production of bioplastic. 119-121.
There are many ways in which starch can be utilized as biopolymer. First, important chemicals like organic acids and ethanol can be produced from starch. Second, it can serve as a filling material to plastics. Third, through modification of starch122,123.Mostly starch is blended with thermoplastic polymers and plasticizers and thermoplastic starch is obtained which is biodegradable and has varied applications. The linear structure of amylose in starch favours the development of bioplastics which are stronger and have highly adjustable mechanical and physical properties. In contrast, the branched structure of amylopectin leads to production of bioplastics which have low resistance to tensile strength and elongation 124. Starch is mainly modified with other materials to exhibit desired properties. Novamont is the most active and major producer of starch-based products in the market 125,126.
Starch and transformed starches possess a wide array of applicability in the production of films for shopping, fishing bait bags, packing materials, food packaging, special mulch films, packaging foam and injection-molded materials, for example, ‘take away’ food containers. Further useful areas of starch applications are textiles, construction, pharmaceuticals, cosmetics, paints, paper and cardboard industries 127.
In certain thermoplastic starches, brittleness arises due to increase in crystallinity, brittleness also increases over time. In future starch can be employed as a natural and renewable substrate for the synthesis of many biopolymers 46.
Cellulose
Cellulose is the foremost constituent of cell walls of almost all plants and a rigid polysaccharide. It is comprised of a large number of monosaccharide units of β-glucose joined in linear fashion. Common sources of making materials for the synthesis of cellulosic plastics are wood and cotton fibres. Plant fibres are dissolved in carbon disulphide and alkalis to form viscose, from which rayon (fibre) or cellophane (film) can be obtained in a bath of sodium sulfate and sulfuric acid 46.
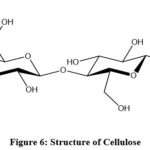 |
Figure 6: Structure of Cellulose. |
Cellulose has high tensile strength and it is a stiff polymer. Because of high abundance, cellulose can be served as a renewable source of substrate for the synthesis of sustainable bioplastic materials 128,129. The important derivatives of cellulose that have industrial uses are cellulose esters, cellulose acetate and regenerated cellulose for fibres 129. Eastman Chemical is one of the chief producer of cellulosic plastics 46.
Due to the structural compatibility of cellulose, it forms hydrogels which found utility in scaffolds of cell culture, tissue engineering, bone implantation, modelling of cartilage, drug delivery, absorbance of heavy metals, retaining of soil water, effective release of fertilizers for agricultural purposes 130.
Cellulose ethers have applications in food, pharmaceuticals, personal care, paint and construction activities. Cellulose esters are widely used for producing films and fibres. Regenerated cellulose fibres are mainly utilized in textiles, disposables items for hygiene and home furnishing fabrics due to their high thermal stability 131,132.
Chitosan and Chitin
Chitosan and Chitin are amplest natural amino polysaccharides and precious bio-resources based natural polymers. After cellulose, chitin is the second most plentiful agro-polymer found in nature. Chitin is a long chain polymer of N-acetylglucosamine. It is present in the exoskeleton of arthropods, cell walls of yeasts and fungi in the form of crystalline microfibrils that constitute their structural components 133. Chitin is non-toxic, bio-compactible and biodegradable in nature 134. These are commercially produced from prawn, crab and shrimp wastes through chemical extraction processes 135,136.
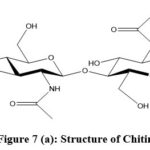 |
Figure 7 (a): Structure of Chitin. |
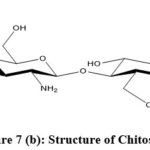 |
Figure 7(b): Structure of Chitosan. |
Chitosan is produced from chitin by deacetylation of chitin. Chitosan has many distinguishable features which include chemical inertness, biodegradability, high mechanical strength, biocompatibility, bioactivity, high mechanical strength, low toxicity, low cost and good film-forming properties 137-139. Based on different properties it has a vast area of applications in pharmaceuticals, cosmetics, plant protection, water treatment, medical devices, skin care industry 140-144.
Bio-based polymers
Polylactic Acid (PLA)
It is a bio-based and biodegradable polymer. It has a monomeric unit, Lactic acid which is produced either by micro-organism catalysed fermentation of simple carbohydrates or by chemical processes 145.
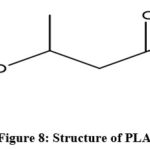 |
Figure 8: Structure of PLA. |
On a global platform, PLA is receiving greater attention from both academia and industries because of technological advances in its production process and functionality 146. The active companies in this field are Nature Works, Teijin, Total Corbion, and Purac which mainly utilize lactic acid fermentation process instead of chemical synthesis 147. The most common raw materials used in this process are corn starch, cassava roots, sugarcane and potatoes as the source of carbohydrates which accounts for the industrial process of lactic acid synthesis renewable and sustainable 148.
PLA as bio-based polymer has many amazing characteristics such as glossy appearance, efficiency to withstand different processing conditions, ability to act as a barrier, good transparency & very high rigidity149.
Polylactic acid is one of the major productive biopolymers that yield 1 kilogram of product from 1.6 kilogram of the fermented carbohydrate source. PLA has greater utility in food-packing industry (for the production of bottles, cups, foil, moulds, etc.), textile industry, cosmetics and pharmaceutical industry, electronics and automotive industry and in 3D printings 127,148. Now-a-days blends of PLA with other polymers are also utilized to intensify its functional and biodegradable properties so as to have advance packaging applications 150.
But the major drawback of PLA is that it is not heat resistant and cannot withstand high temperatures. High brittleness, low toughness and slow biodegradation are also some of the problems associated with PLA which further hamper its specific applications. Research is being done to develop PLA as more eco-friendly and to make it heat resistant 151.
Polyhydroxy Alkanoates (PHAs)
PHAs are a broad group of biodegradable polymers, but mainly refer to poly (3-hydroxy butyrate) and its copolymer (poly (3-hydroxy butyrate-co-3-hydroxy valerate)).
On the basis of carbon chain length of the monomers, PHAs are grouped into two major categories:
short chain length 3-3-hydroxyalkanoates (scl-3HA) with 3-5 carbon atoms
medium chain length-3-hydroxyalkanoates (mcl-3HA) having 6-16 carbon atoms 152.
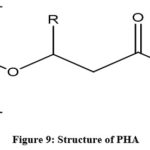 |
Figure 9: Structure of PHA. |
Metabolix is the main leading company in the production of PHAs.A large number of patents associated with PHA shows that it is used extensively as a biodegradable polymer153.
These polyoxoesters are synthesized by bacteria through anaerobic digestion of lipids or sugars via polyhydroxy fatty acids. Polyhydroxy alkanoates (PHAs) are produced in bacterial cells as energy storage product. These are separated from bacterial cells through centrifugation, filtration and solvent extraction. Food and agricultural wastes such as mango peel, potato peel, wheat bran, rice husk, straw, etc. are also utilized in the production of PHAs.
Main properties of PHAs are 154:
Completely biodegradable in water, soil and compost
Highly resistant to oil and grease
Good printability
High thermal stability (can withstand temperatures nearly upto 180̊ C)
Non toxicity
Biocompatibility
Resistance to hydrolytic degradation
PHAs are mostly used in packaging, disposable cups, disposable razors, shampoo bottles, surgical pins, surgical stitches, disposable knives and forks, injection molding grades and woven medical patches 126. With a greater area of applications, the issues that hinder the commercialization of PHAs are slow crystallization rate, brittleness, narrow processing window and sensitivity to thermal degradation 155.
Biocompatibility and mechanical features of PHAs can be altered by adjusting the surface, blending or integrating with different enzymes, polymers and inorganic substances, which further allows a vast range of applications 156,157.
Polybutylene succinate (PBS)
PBS is a biodegradable and aliphatic polymer which is prepared by condensation between 1,4-butanediol and succinic acid. It is synthesized either by monomers produced from fossil-based sources or by fermentation through bacteria. Nowadays, products of 100% bio-PBS are available in the market. Bio-PBS is prepared from bio-succinic acid and bio-ethanol which can be obtained from sugarcane and cassava monomers 158.
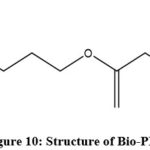 |
Figure 10: Structure of Bio-PBS. |
The route through fermentation is more advantageous as it utilizes renewable resources and energy efficient as compared to chemical process. This bio-based process of synthesis involves utilization of glucose to produce succinic acid and 1,4-butanediol from natural and renewable resources 159.
PBS is a semi-crystalline polymer and its thermal and mechanical characteristics depend on the degree of crystallinity and structure of the crystal 160. PBS has high flexibility, heat resistance, natural fibre compatibility and biodegradability.It has good impact strength and tensile strength along with moderate hardness and rigidity. Due to these properties, they have vast area of applications in industries such as pharmaceutical, agricultural, packaging, textile, electronics and electrical sand automobiles. It is also used for manufacturing plastic utensils, plates, bowls, etc. 161.
The comparatively poor mechanical variability limits the applications of 100% bio-PBS products. So, it has to be copolymerized with certain other polymers for improving its strength and properties 158.
Bio-polyethylene (Bio-PE)
PE is made up of long chain polymers of ethylene which is synthesized as either HDPE (High Density Polyethylene) or LDPE (Low Density Polyethylene) or LLDPE (Linear Low-Density Polyethylene). In chemical industries, PE is produced by polymerization of ethane 162.
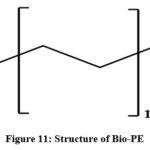 |
Figure 11: Structure of Bio-PE. |
Bio-PE has similar physical, chemical, technical and mechanical properties to polyethylene 163. Ethanol synthesized by microbial fermentation is used in preparing green polyethylene. The production of bio-ethanol utilizes renewable resources such as sugarcane, starch crops including wheat, corn, maize, etc. and other plant wastes 164,165. Bio-PE is used to obtain valuable products by considering eco-friendly methods and hence contributes towards sustainable environment. Brazil’s Braskem company is one of the world’s chief producer of bio-PE 166.
HDPE is the most widely used copolymer of bio-PE due to its significant properties. It has close packed polymer chains which results in strong intermolecular forces that enhance its rigidity and crystallinity. It is highly tough polymer and has good thermal resistance and can bear high temperatures upto 120°C, without having any effect on properties of the material. HDPE is inert to many chemicals, oils, acids, bases, aldehydes, alcohols and esters. Bio-PE has good performance and is also cost effective. It is extensively used in packaging (food packaging which involves packaging of water bottles, milk and plastic bags, carrying bags, plastic bottles and films), personal care, healthcare, laboratory objects, cosmetics, toys, engineering, automotive parts, agriculture and many day-to-day essential commodities.
Even though it has widespread applications in almost all areas but the major disadvantage is that it is non-biodegradable. Because of this it persists in the environment for several hundred years. Attempts are being made to recycle Bio-PE to resolve the problem of non-biodegradability 46.
Bio-polyethlene terephthalate (Bio-PET)
PET is a polyester polymer. The monomer of PET, ethylene terephthalate (bis(2-hydroxyethyl) terephthalate) is prepared by esterification reaction between ethylene glycol and terephthalic acid. It generally contains 30% ethylene glycol (EG) and 70% terephthalic acid (TPA). Bio-PET contains the renewable monomer i.e., monoethylene glycol (MEG). MEG is obtained from sugarcane-derived ethylene. These monomers are derived from fermentation of glucose 167. Researchers have also developed feasible process to synthesize bio-based TPA by utilizing n-butanol, isobutanol, isobutylene, limonene, muconic acid, carbohydrates (fructose and glucose) and terpenes, as substrates 168.
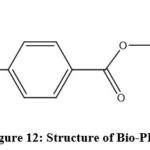 |
Figure 12: Structure of Bio-PET. |
The potential of Bio-PET packaging is similar to other petro-based PET products 169. It has excellent thermal properties. It can act as a good barrier agent 170.
Bio-PET is assisted by Coca Cola by the name ‘Plantbottle’. Sprite, Dasani, and Fresca also use similar packaging for bottles 171. Pepsi has also announced 100% renewable PET material synthesized from pine bark, switchgrass and corn husks. It is mainly used as packing material for bottles. It can be degraded by bacteria Nocardia with its esterase enzyme 172-174.
Comparative Analysis of different types of Bioplastics
The bioplastics vary in accordance to their availability, nature, structure, properties such as thermal stability, biodegradability, tensile strength, etc. A comparative analysis of both natural and bio-based polymers has been done in the following tables:
Table 2: Comparative Analysis of Natural Polymers
|
|
Starch |
Cellulose |
Chitin |
Chitosan |
|
Source |
Stored in plants as their food |
Found in cell wall of plants |
Exoskeleton of arthropods, cell walls of yeasts and fungi |
Chitin |
|
Structural Unit |
α- glucose |
β-glucose |
N-acetylglucosamine |
N-glucosamine and N-acetylglucosamine |
|
Nature |
Occurs naturally |
Occurs naturally |
Occurs naturally |
Obtained by deacetylation of Chitin |
|
Biodegradability |
Yes |
Yes |
Yes |
Yes |
|
Thermal Stability |
Good |
High |
High |
Good |
|
Tensile Strength |
Good |
High |
High |
Low to high |
|
Toxicity |
Non toxic |
Non toxic |
Non toxic |
Non toxic |
|
Colour |
White |
White |
Yellow-white |
Yellow |
|
Renewability |
Yes |
Yes |
Yes |
Yes |
Table 3: Comparative Analysis of Bio-Based Polymers.
|
|
PLA |
PHA |
Bio-PBS |
Bio-PE |
Bio-PET |
|
Source |
Corn starch, cassava roots, sugarcane and potatoes as the source of carbohydrates |
Bacteria |
Sugarcane and cassava monomers |
Bio-ethanol obtained from glucose |
Glucose and cellulose biomass |
|
Structural Unit |
Lactic acid |
Hydroxy alkanoates |
Succinic acid and 1,4-butanediol |
Ethylene |
Ethylene glycol and terephthalic acid |
|
Nature |
Synthesized |
Synthesized |
Synthesized |
Synthesized |
Synthesized |
|
Biodegradability |
Yes but slow |
Yes |
Yes |
No |
May or may not be |
|
Thermal Stability |
Low to Medium |
Low to Medium |
High |
Medium |
High |
|
Tensile Strength |
Good |
Good |
Good |
Good |
High |
Future scope
The area of exploration for biopolymers is significantly expanding because of its biodegradability and sustainability. Bioplastics is a wide-ranging family of materials with extensively diversified properties. Bioplastic products have a vast area of applicability from day-to-day essential commodities to advance technological applications.
But economy scale including management of bio-based raw materials, conduct of the raw materials and their cost of manufacturing are some of the challenges that need to be addressed in near future.
To design these processes economically accessible, it is very essential to evolve
logistics for renewable feedstocks,
novel synthetic approaches by restoring existing techniques with enhanced yields,
novel strains of microbes and enzymes and
effective ways for the recycling of bio-based raw materials.
Innovations in elementary reforming of bioplastics with enhanced economies aimed to recycling will open up new opportunities in the area of development of sustainable bioplastics.
Conclusion
Alarming rate of environmental pollution caused by synthetic polymers made the search necessary for developing sustainable and cost-effective alternatives. In this series, emergence of bioplastics has become a novel and an innovative field of research for scientists all over the world. Bioplastics act as eco- friendly, highly biodegradable and cost-efficient alternatives to the conventional petro-based plastics.A rational design of bioplastics to bestow specific and desired properties such as functionality, biodegradability, recyclability and utilization of unaccounted biomass as a precious and renewable resource would together manifest a sustainable and greener approach towards production and effective applications of bioplastics.
Acknowledgment
Authors acknowledge the research facilities from the Head, Department of Chemistry, University of Rajasthan, Jaipur during the research work.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Sources
There is no funding Soure.
References
- Lackner, M.; Othmer, K.; Encyclopedia of Chemical Technology, John Wiley and Sons, New York, 2015.
- Alvarez-Chavez, C. R.; Edwards, S.; Moure-Eraso, L.; Geiser, K. J. Clean. Prod. 2012,23 (1), 47-56.
CrossRef - Narissara, K.; Shabbir, H. G. J. Sustain. Energy Environ. 2013,4, 15-21.
- Spierling, S.; Knuffer, E.; Behnsen, H.; Mudersbach, M.; Krieg, H.; Springer, S.; Albrecht, S.; Hermann, C.; Endres, H. J. Clean. Prod. 2018, 185, 476-491. https://doi.org/10.1016/j.jclepro.2018.03.014
CrossRef - Gill, M. Int. J. Res. Appl. Nat. Soc. Sci. 2014, 2, 115
- Hottle, T. A.; Bilec, M. M.; Landis, A. E. Polym. Degrad. Stab. 2013, 98,1898-1907. https://doi.org/10.1016/j.polymdegradstab.2013.06.016
CrossRef - Wang, Y.; Sun, Z.; Tian, J.; Wang, H.; Wang, H.; Ji, Y. Mater. Sci. 2016, 22, 290-294.
CrossRef - Thakur, S.; Verma, A.; Sharma, B.; Chaudhary, J.; Tamulevicius, S.; Thakur, V. K. Curr. Opin. Green Sustain. Chem. 2018, 13, 68-75.
CrossRef - Al-Salem, S. M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. J. Environ. Manage. 2017, 197, 177-198.
CrossRef - Sharuddin, S. D. A.; Abnisa, F.; Daud, W. M. A. W.; Aroua, M. K. Energy Convers. Manag. 2016, 115, 308-326.
CrossRef - Napper, I. E.; Thompson, R. C. Environ. Sci. Technol. 2019, 53 (9), 4775-4783. https:// doi.org/10.1021/acs.est.8b06984
CrossRef - Albuquerque, P. B.; Malafaia, C. B. Int. J. Biol. Macromol. 2018, 107(Part A) 615-625. https://doi.org/10.1016/j.ijbiomac.2017.09.026
CrossRef - Shafqat, A.; Tahir, A.; Mahmood, A.; Pugazhendhi, A. Biocatal. Agric. Biotechnol. 2020, 27, 101540
CrossRef - Paul, S.; Sen, B.; Das, S.; Abbas, S. J.; Pradhan, S. N.; Sen, K.; Ali, S. I. Int. J. Environ. Anal. Chem. 2021, 1
CrossRef - Narancic, T.; Verstichel, S.; Chaganti, S. R.; Morales-Gamez, L.; Kenny, S. T.; De Wilde, B.; Padamati, R. B.; O’Connor, K. E. Environ. Sci. Technol. 2018, 52, 10441-10452.
CrossRef - Sharma, C.; Manepalli, P. H.; Thatte, A.; Thomas S, Kalarikkal N and Alavi S 2017 Colloid Polym. Sci. 295 1695-1708.
CrossRef - Saygin, H.; Baysal, A. Bull. Environ. Contam. Toxicol. 2020, 105,26-35. https://doi.org/10.1007/s00128-020-02993-9
CrossRef - Jeremic, S.; Milovanovic, J.; Mojicevic, M.; Skaro Bogojevic, S.; Nikodinovic-Runic, J.; J. Serb. Chem. Soc. 2020, 85(1), 1507-1538.
CrossRef - Unmar, G.; Mohee, R. R. Bioresour. Technol. 2008, 99(15), 6738-6744. https://doi.org/10.1016/j.biortech.2008.01.016
CrossRef - Philip, J. C.; Ritchie, R. J.; Guy, K.; Trends Biotechnol. 2013, 31 (2), 65-67. https://doi.org/10.1016/j.tibtech.2012.11.009
CrossRef - Nikolaivits, E.; Pantelic, B.; Azeem, M.; Taxeidis, G.; Babu, R.; Topakas, E.; Fournet, M. B.; Nikodinovic-Runic, J. Front. Bioeng. Biotechnol. 2021, 9
CrossRef - Emadian, S. M.; Onay, T. T.; Demirel, B. Waste Manag. 2017, 59, 526-536. https://doi.org/10.1016/j.wasman.2016.10.006
CrossRef - Anastas, P. T.; Kirchhoff, M. M. Acc. Chem. Res. 2002, 35, 686-694. https://doi.org/10.1021/ar010065m
CrossRef - Sanyang, M. L.; Sapuan, S. M.; Jawaid, M.; Ishak, M. R.; Sahari, J. J. Food Sci. Technol. 2016, 53 (1), 326-336. https://doi.org/10 .1007/s13197-015-2009-7
CrossRef - Thompson, R.; Moore, C.; Andrady, A.; Gregory, M.; Takada, H.; Weisberg, S. Science 2005, 310, 1117
CrossRef - Barnes, D. K. A.; Galgani, F.; Thompson, R. C.; Barlaz, M. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364 (1526), 1985-1998.
CrossRef - Wang, J.; Tan, Z.; Peng, J.; Qiu, Q.; Li, M. Mar. Environ. Res. 2016, 113, 7-17. https://doi.org/10.1016/j.marenvres.2015.10.014
CrossRef - Ghimire, S.; Flury, M.; Scheenstra, E. J.; Miles, C. A. Sci. Total Environ. 2020, 703,135577
CrossRef - North, E. J.; Halden, R. U. Rev. Environ. Health 2013, 28(1), 1-8. https://doi.org/10.1515/reveh-2012-0030
CrossRef - Klein, F.; Emberger-Klein, A.; Menrad, K.; M¨ohring, W.; Blesin, J. M. Sustain. Prod. Consum. 2019, 19, 33-43. https://doi.org/10.1016/j. spc.2019.01.004
CrossRef - Lu, L.; Luo, T.; Cai, C.; Fu, Z.; Jin, Y. Sci. Total Environ. 2019, 667(8), 94-100. https://doi.org/10.1016/j.scitotenv.2019.02.380
CrossRef - Gregory, M. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2013-2025. https://doi.org/10.1098/rstb.2008.0265
CrossRef - Rochman, C. M.; Browne, M. A.; Underwood, A. J.; van Franeker, J. A.; Thompson, R. C.; Amaral-Zettler, L. A. Ecology 2016, 97,302-312.
CrossRef - Wilcox, C.; Mallos, N. J.; Leonard, G. H.; Rodriguez, A.; Hardesty, B. D. Mar. Policy 2016, 5, 107-114.
CrossRef - Rochman, C. M. Science 2018, 360(6384), 28-29.
CrossRef - Lebreton, L.; Slat, B.; Ferrari, F.; Sainte-Rose, B.; Aitken, J.; Marthouse, R.; Hajban, S.; Cunsolo, S.; Schwartz, A.; Levivier, A.; Noble, K.; Debeljak, P.; Maral, H.; Schoeneich-Argent, R.; Brambini, R.; Reisser, J. Sci. Rep. 2018, 8 (1),1-15.
CrossRef - Gironi, F.; Piemonte, V. Energ. Source. Part A 2011, 33, 1949-1959. https://doi.org/10.1080/15567030903436830
CrossRef - Skogen, K.; Helland, H.; Kaltenborn, B. J. Nat. Conserv. 2018, 44, 12-20. https://doi.org/ 10.1016/j.jnc.2018.06.001
CrossRef - Rahimi, A.; Garcia, J. M. Nat. Rev. Chem. 2017, 1, 0046
CrossRef - Sayyed, R. Int. J. Biotechnol. Biosci. 2012, 2(3), 187-189.
- Scalenghe, R. Heliyon 2018, 4(12), e00941.
CrossRef - Li, W. C.; Tse, H. F.; Fok, L. Sci. Total Environ. 2016, 566-567, 333-349. https://doi.org/10.1016/j.scitotenv.2016.05.084
CrossRef - Azahari, N. A.; Othman, N.; Ismail, H. J. Phys. Sci. 2011, 22 (2), 15-31.
- Tharanathan, R. N. Trends Food Sci. Technol. 2003, 14, 71-78.
CrossRef - Santana, I.; Felix, M.; Guerrero, A.; Bengoechea, C. Polymers 2022, 14, 355 https://doi.org/10.3390/polym14020355
CrossRef - Babu, R. P.; O’Connor, K.; Seeram, R. Prog. Biomater. 2013, 2(1), 8 https://doi.org/10.1186/2194-0517-2-8
CrossRef - Bharti, S. N.; Swetha, G. J. Pet. Environ. Biotechnol. 2016,7, 272 https://doi.org/10.4172/2157-7463.1000272
CrossRef - Chong, T. Y.; Law, M. C.; Chan, Y. S. J. Polym. Environ. 2021, 29(2), 363-381. https://doi.org/10.1007/s10924-020-01888-4
CrossRef - Marichelvam, M. K.; Jawaid, M.; Asim, M. Fibres 2019, 7(32), 1 https://doi.org/10.3390/fib7040032
CrossRef - Peelman, N.; Ragaert, B.; De Meulenaer, Adons, D.; Peeters, R.; Cardon, L.; Impe, F.; Vand Devlieghere, F. Trends Food Sci. Technol. 2013, 32(2), 128-141. https://doi.org/10.1016/j.tifs.2013.06.003
CrossRef - Sinan, M. Curr. World Environ. 2020, 15 (1), 24-28.
CrossRef - Bilo, F.; Pandini, S.; Sartore, L.; Depero, L. E.; Gargiulo, G.; Bonassi, A.; Federici, S.; Bontempi, E. J. Clean. Prod. 2018, 200, 357-368.
CrossRef - Williams, J. H.; De Benedictis, A.; Ghanadan, R.; Mahone, A.; Moore, J.; Morrow III, W. R.; Price, S.; Torn, M. S. Science 2012, 335,53-59. John Wiley & Sons Inc
CrossRef - Davis, G.; Song, J. H. Ind. Crops Prod. 2006, 23, 147-161. https://doi.org/10.1016/J.INDCROP.2005.05.004
CrossRef - Sankauskaite, A.; Stygiene, L.; Tumeniene, M. D.; Krauledas, S.; Jovaisiene, L.; Puodziuniene, R. Mater. Sci. 2014, 20,189-192.
CrossRef - Vasava, D. V.; Shah, T. V. e-Polym. 2019, 19(1), 385-410.
CrossRef - Avella, M.; De Vlieger, J. J.; Errico, M. E.; Fischer, S.; Vacca, P.; Vope, M. G. Food Chem. 2009, 93, 548-558.
- Chek, M. F.; Kim, S. Y.; Mori, T.; Tan, H. T.; Sudesh, K.; Hakoshima, T. iScience 2020,101084.
CrossRef - Zhu, J.; Wang, C. Mar. Pollut. Bull. 2020, 161, 111774 https://doi.org/10.1016/j.marpolbul.2020.111774
CrossRef - Varsha, Y. M.; Savitha, R. J Microb. Biochem. Technol. 2011, 3, 099-105. https://doi.org/10.4172/1948-5948.1000059
CrossRef - Khodaei, D.; Hamidi-Esfahani, Z.; Lacroix, M. Food Biosci. 2020, 36, 100660 https://doi.org/10.1016/j.fbio.2020.100660
CrossRef - Ezeonu, C. S.; Ezeonu, N. C. J. Pet. Environ. Biotechnol. 2016, 7, 301 https://doi.org/10.4172/2157-7463.1000301
CrossRef - Ezeonu, C. S.; Temitope, D. F. Adv. Biotechnol. Microbiol. 2018, 10 (5), 0096 https://doi.org/10.19080/AIBM.2018.10.555797
CrossRef - Hubbe, M. A.; Lavoine, N.; Lucia, L. A.; Dou, C. Bioresources 2021, 16(1), 2021-2083
CrossRef - Panchal, S. S.; Vasava, D. V. ACS Omega 2020, 5(9), 4370-4379. https://doi.org/10.1021/acsomega.9b04422
CrossRef - Karan, H.; Funk, C.; Grabert, M.; Oey, M.; Hankamer, B. Trends Plant Sci. 2019, 24,237-249.
- Mohanty, A. K.; Misra, M.; Drzal, L. T. J. Polym. Environ. 2002, 10(1), 19-26. https://doi.org/10.1023/A:1021013921916
CrossRef - Moshood, T. D.; Nawanir, G.; Mahmud, F.; Mohamad, F.; Ahmad, M. H.; Ghani, A. A. CRGSC 2022, 5, 100273
CrossRef - Moshood, T. D.; Nawanir, G.; Mahmud, F.; Mohamad, F.; Ahmad, M. H.; Ghani, A. A. Clean. Eng. Technol. 2022, 6, 100404
CrossRef - Sintim, H. Y.; Bandopadhyay, S.; English, M. E.; Bary, A. I.; DeBruyn, J. M.; Schaeffer, S. M.; Miles, C. A.; Reganold, J. P.; Flury, M. Agric. Ecosyst. Environ. 2019, 273 (1), 36-49.
CrossRef - Boisvert, A.; Jones, S.; Issop, L.; Erythropel, H. C.; Papadopoulos, V.; Culty, M. Environ. Res. 2016, 150, 496-512.
CrossRef - Moshood, T. D.; Adeleke, A. Q.; Nawanir, G.; Ajibike, W. A.; Shittu, R. A. Int. J. Recent Technol. Eng. 2020, 9 (1), 1627-1634.
CrossRef - Shamsuddin, I. M.; Jafar, J. A.; Shawai, A. S. A.; Yusuf, S.; Aminu, M. L. I. Adv. Biosci. Bioeng. 2017, 5, 63-70.
CrossRef - Vert, M.; Doi, Y.; Hellwich, K. H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaud, M.; Schue, F. Pure Appl. Chem. 2012, 84, 377-410.
CrossRef - Song, J. H.; Murphy, J. H.; Narayan, R.; Davies, G. B. H. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2127-2139.
CrossRef - Vea, E. B.; Romeo, D.; Thomsen, M. Procedia CIRP 2018, 69, 591-596. https://doi.org/10.1016/j.procir.2017.11.062
CrossRef - Simonic, M.; Zemljic, F. Chem. Ind. Chem. Eng. Q. 2020, 27, 26 https://doi.org/10.2298 CICEQ191024026S
CrossRef - Karbowiak, T.; Debeaufort, F.; Voilley, A. Food Hydrocoll. 2007, 21, 879-888. https://doi.org/10.1016/j.foodhyd.2006.07.017
CrossRef - Averous, L. J. Macromol. Sci. Part C Polym. Rev. 2004, 44, 231-274. https://doi.org/10.1081/MC-200029326
CrossRef - Alvarez, S.; Weng, S.; Alvarez, C.; Marcet, I.; Rendueles, M.; Diaz, M. Food Packag. Shelf Life 2021, 28, 100639
CrossRef - Galus, S.; Kadzinska, J. Trends Food Sci. Technol. 2015, 45, 273-283. https://doi.org/10.1016/j.tifs.2015.07.011
CrossRef - Yu, P. H.; Chua, H.; Huang, A. L.; Ho, K. P. Appl. Biochem. Biotechnol. 1999, 78, 445-454.
CrossRef - Muthuraj, R.; Mekonnen, T. Polymers 2018, 145, 348-373. https://doi.org/10.1016/j.polymer.2018.04.078
CrossRef - Rosenboom, J. G.; Langer, R.; Traverso, G. Nat. Rev. Mater. 2022, 7, 117-137. https://doi.org/10.1038/s41578-021-00407-8
CrossRef - Hatti-Kaul, R.; Nilsson, L. J.; Zhang, B.; Rehnberg, N.; Lundmark, S. Trends Biotechnol. 2020, 38 (1), 50-67.
CrossRef - Garcia, J. M.; Robertson, M. L. Science 2017, 358 (6365), 870-872. https://doi.org/10.1126/science.aaq0324
CrossRef - Narancic, T.; O’Connor, K. E. Microb. Biotechnol. 2017, 10(5), 1232-1235. https://doi.org/10.1111/1751-7915.12775
CrossRef - Imre, B.; Pukanszky, B. Eur. Polym. J. 2013, 49, 1215-1233. https://doi.org/10.1016/j.eurpolymj.2013.01.019
CrossRef - Schneiderman, D. K.; Hillmyer, M. A. Macromolecules 2017, 50 (10), 3733-3749. https://doi.org/10.1021/acs.macromol.7b00293
CrossRef - Ahmed, S. T.; Leferink, N. G. H.; Scrutton, N. S. Mol. Catal. 2019, 467, 95-110. https://doi.org/10.1016/j.mcat.2019.01.036
CrossRef - Zhang, X.; Fevre, M.; Jones, G. O.; Waymouth, R. M. Chem. Rev. 2018, 118 (2), 839-885.
CrossRef - Nafchi, A. M.; Moradpour, M.; Saeidi, M.; Alias, A. K. Starch-Starke 2013, 65, 61-72. https://doi.org/10.1002/star.201200201
CrossRef - Abe, M. M.; Martins, J. R.; Sanvezzo, P. B.; Macedo, J. V.; Branciforti, M. C.; Halley, P.; Botaro, V. R.; Brienzo, M. Polymers 2021, 13, 2484 https:// doi.org/10.3390/polym13152484
CrossRef - Haider, T. P.; Volker, C.; Kramm, J.; Landfester, K.; Wurm, F. R. Angew. Chem. Int. Ed. 2019, 58 (1), 50-62.
CrossRef - Reddy, R. L.; Reddy, V. S.; Gupta, G. A. Int. J. Emerg. Technol. Adv. Eng. 2013, 3 (5), 76-81.
- Arikan, E. B.; Ozsoy, H. D. J. Civ. Eng. Arch. 2015, 9, 188-192. https://doi.org/10.17265/1934-7359/2015.02.007
CrossRef - Chen, Y. J. J. Chem. Pharm. Res. 2014, 6(1), 226-231.
- Yu, J.; Chen, L. X. L. Environ. Sci. Technol. 2008, 42, 6961-6966.
CrossRef - Scaffaro, R.; Botta, L.; Maio, A.; Mistretta, M. C.; La Mantia, F. P. Materials 2016, 9(5), 351
CrossRef - Zoungranan, Y.; Lynda, E.; Dobi-Brice, K. K.; Tchirioua, E.; Bakary, C.; Yannick, D. D. J. Environ. Chem. Eng. 2020, 8 (5), 104396
CrossRef - Kale, G.; Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S. E.; Singh, S. P. Macromol. Biosci. 2007, 7,255-277.
CrossRef - Cinar, S. O.; Chong, Z. K.; Kucuker, M. A.; Wieczorek, N.; Cengiz, U.; Kuchta, K. Int. J. Environ. Res. 2020, 17(11), 3842
CrossRef - Kfoury, G.; Raquez, J. M.; Hassouna, F.; Odent, J.; Toniazzo, V.; Ruch, D. Front. Chem. 2013, 1 (32), 1
CrossRef - Chaudhary, A. L.; Torley, P. J.; Halley, P. J.; McCaffery, N.; Chaudhary, D. S. Carbohydr. Polym. 2009, 78, 917-925.
CrossRef - El Miri, N.; Abdelouahdi, K.; Barakat, A.; Zahouily, M.; Fihri, A.; Solhy, A.; El Achaby, M. Carbohydr. Polym. 2015, 129, 156-167.
CrossRef - Pang, M. M.; Pun, M. Y.; Ishak, Z. A. M. J. Appl. Polym. Sci. 2013, 129, 3656-3664. https://doi.org/10.1002/app.39123
- Khan, B.; Niazi, M. K. N.; Samin, G.; Jahan, Z. J. Food Process Eng. 2016, 40, e12447
- Ali, A.; Yu, L.; Liu, H.; Khalid, S.; Meng, L.; Chen, L. J. Appl. Polym. Sci. 2017, 134 (32), 45159.
- Niranjana Prabhu, T.; Prashantha, K. Polym. Compos. 2018, 39 (7), 2499-2522. https://doi.org/10.1002/pc.24236
- Udeni Gunathilake, T. M. S.; Ching, Y. C.; Chuah, C. H.; Sabariah, J.; Lin, P. C. Materials 2016, 9 (12), 991
- Agustin, M. B.; Ahmmad, B.; Alonzo, S. M. M.; Patriana, F. M. J. Reinf. Plast. Compos. 2014, 33, 2205-2213.
- Griffin, G. J. L. Polym. Degrad. Stab. 1994, 45, 241-247.
- Araujo, M. A.; Cunha, A.; Mota, M. Biomaterials 2004, 25, 2687-2693. https://doi.org/10.1016/j.biomaterials.2003.09.093
- Zhang, J. F.; Sun, X. Z. Biomacromolecules 2004, 5, 1446-1451. https://doi.org/10.1021/bm0400022
- Hern-andez-Jaimes, C.; Meraz, M.; Lara, V. H.; Gonz-alez-Blanco, G.; Buendia-Gonz-alez, L. Rev. Mex. Ing. Quim. 2017, 16 (1), 169-178.
- Gadhave, R. V.; Das, A.; Mahanwar, P. A.; Gadekar, P. T. Open J. Polym. Chem. 2018, 8, 21-33.
- Orenia, R. M.; Collado III, A.; Magno, M. G.; Cancino, L. T. Asian J. Multidiscip. Stud. 2018, 1 (1), 61-77.
- Che, L.; Li, D.; Wang, L. J.; Chen, X. D.; Mao, Z. H. Int. J. Food Prop. 2007, 10, 527-536.
- Cunha, A. G.; Gandini, A. Cellulose 2010, 17, 1045-1065.
- Thakore, I. M.; Desai, S.; Sarawade, S.; Devi, S. Eur. Polym. J. 2001, 37, 151-160. https://doi.org/10.1016/S0014-3057(00)00086-0
- Yang, J.; Ching, Y. C.; Chuah, C. H.; Hai, N. D.; Singh, R.; Nor, A. R. M. Cellulose 2021, 28, 4191-4210.
- Amin, M. R.; Chowdhury, M. A.; Kowser, M. A.; Heliyon 2019, 5(8), 1 e02009 https://doi.org/10.1016/j.heliyon.2019.e02009
- Li, J.; Ye, F.; Liu, J.; Zhao, G. Food Hydrocoll. 2015,46, 226-232. https://doi.org/10.1016/j.foodhyd.2014.12.017
- Fakhouri, F. M.; Martelli, S. M.; Caon, T.; Velasco, J. I.; Mei, L. H. I. Postharvest Biol. Technol. 2015, 109, 57-64.
- Li, S.; Juliane, H.; Martin, K. P. PRO-BIP 2009, 1, 1
- Jariyasakoolroj, P.; Leelaphiwat, P.; Harnkarnsujarit, N.; J. Sci. Food Agric. 2020, 100 (14), 5032-5045.
- Peresin, M. S.; Habibi, Y.; Zoppe, J. O.; Pawlak, J. J.; Rojas, O. J. Biomacromolecules 2010, 11(3), 674-681.
- Moon, R. J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Chem. Soc. Rev. 2011, 40, 3941-3994.
- Chmielarz, P. Express Polym. Lett. 2017, 11(2), 140-151. https://doi.org/10.3144/expresspolymlett.2017.15
- Kamel, S.; Ali, N.; Jahagir, K.; Shah, S. M.; El-Gendy, Express Polym. Lett. 2008, 2(11), 758-778.
- Kevin, J. E.; Charles, M. B.; John, D. S.; Paul, A. R.; Brian, D. S.; Michael, C. S.; Debra, T. Prog. Polym. Sci. 2001, 26 (9), 1605-1688.
- Rinaudo, M. Prog. Polym. Sci. 2006, 31 (7), 603-632. https://doi.org/10.1016/j.progpolymsci.2006.06.001
- Itoh, Y.; Kawase, T.; Nikaidou, N.; Fukada, H.; Mitsutonii, M.; Itoh, Y. Biosci. Biotechnol. Biochem. 2002, 66(5), 1084-1092.
- Flieger, M.; Kantorova, M.; Prell, A.; Rezanka, T.; Votruba, J. Folia Microbiol. 2003, 48 (1), 27-44.
- Xu, Y.; Gallert, C.; Winter, J. Appl. Microbiol. Biotechnol. 2008, 79, 687-697. https://doi.org/10.1007/s00253-008-1471-9
- Verlee, A.; Mincke, S.; Stevens, C. V. Carbohydr. Polym. 2017, 164, 268-283. https://doi.org/10.1016/j.carbpol.2017.02.001
- Epure, V.; Griffon, M.; Pollet, E.; Averous, L. Carbohydr. Polym. 2011, 83 (2), 947-952.
- Liu, M.; Zhang, Y.; Wu, C.; Xiong, S.; Zhou, C. Int. J. Biol. Macromol. 2012, 51(4), 566-575.
- Ramya, R. V.; Jayachanndran, K. S.; Sudha, P. N. J. Biomater. Tissue Eng. 2012, 2(2), 100-111.
- Kumar, M. N. V. R. React. Funct. Polym. 2000, 46(1), 1-27.
- Bae, K. P.; Moo-Moo, K. Int. J. Mol. Sci. 2010, 11(12), 5152-5164. https://doi.org/10.3390/ijms11125152
- Bansal, V.; Sharma, P. K.; Sharma, N.; Pal, O.; Malviya, R. Adv. Biol. Res. 2011, 5(1), 28-37.
- Ke, C. L.; Deng, F. S.; Chuang, C. Y.; Lin, C. H. Polymers 2021, 13(6), 904
- Doran-Peterson, J.; Cook, M. D.; Sarah, K. Plant J. 2008, 54(4), 582-592. https://doi.org/10.1111/j.1365-313X.2008.03480.x
- Helanto, K. E.; Matikainen, L.; Talja, R.; Rojas, O. J. Bioresources 2019, 14(2), 4902-4951.
- Jamshidian, M.; Tehrany, E. A.; Imran, M.; Jacquot, M.; Desobry, S. Compr. Rev. Food Sci. Food Saf. 2010, 9(5), 552-571.
- Wang, Y.; Tashiro, Y.; Sonomoto, K. J. Biosci. Bioeng. 2015, 119(1), 10-18. https://doi.org/10.1016/j.jbiosc.2014.06.003
- Nakajima, H.; Dijkstra, P.; Loos, K. Polymers 2017, 9 (10), 523
- Wu, H.; Wen, B.; Zhou, H.; Zhou, J.; Yu, Z.; Cui, L.; Huang, T.; Cao, F. Polym. Degrad. Stab. 2015, 121, 100-104.
- Su, S.; Kopitzky, R.; Tolga, S.; Kabasci, S. Polymers 2019, 11,1193 https://doi.org/10.3390/polym11071193
- Kaniuk, L.; Stachewicz, U. ACS Biomater. Sci. Eng. 2021, 7, 5339-5362.
https://doi.org/10.1021/acsbiomaterials.1c00757 - Elvers, D.; Song, C. H.; Steinbuchel, A.; Leker, J. Polym. Rev. 2016, 56, 584-606. https://doi.org/10.1080/15583724.2015.1125918
- Chen, G. Q. Chem. Soc. Rev. 2009, 38,2434-2446.
- Hong, S. G.; Gau, T. K.; Huang, S. C. J. Therm. Anal. Calorim. 2011, 103, 967-975. https://doi.org/10.1007/s10973-010-1180-3
- Anjum, A.; Zuber, M.; Zia, K. M.; Noreen, A.; Anjum, M. N.; Tabasum, S. Int. J. Biol. Macromol. 2016, 89,161-174.
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Adv. Ind. Eng. Polym. Res. 2020, 3,27-35. https://doi.org/10.1016/j.aiepr.2019.11.002
- Cheroennet, N.; Pongpinyopap, S.; Leejarkpai, T.; Suwanmanee, U. J. Clean. Prod. 2016, 167, 987-1001.
- Bechthold, I.; Bretz, K.; Kabasci, S.; Kopitzky, R.; Springer, A. Chemical Eng. Technol. 2008, 31, 647-654.
- Nicolas, J.; Floriane, F.; Francoise, F.; Alan, R.; Jean, P. P.; Patrick, F.; Rene, S. L. J. Polym. Sci. Part A: Polym. Chem. 2011, 49(24), 5301-5312.
- Jian-Bing, Z.; Ling, J.; Yi-Dong, L.; Madhusudhan, S.; Tao, L.; Yu-Zhong, W. Carbohydr. Polym. 2011, 83 (2), 762-768.
- Danso, D.; Chow, J.; Streit, W. R. Appl. Environ. Microbiol. 2019, 85, 01095-19. https://doi.org/10.1128/AEM.01095-19
- Mendieta, C. M.; Vallejos, M. E.; Felissia, F. E.; Chinga‑Carrasco, G.; Area, M. C. J. Polym. Environ. 2010, 28, 1-16.
- Luiz, A.; De Castro, R.; Morschbacker, US Patent 2010/0069691A1 2010
- Guangwen, C.; Shulian, L.; Fengjun, J.; Quan, Y. Catal. Today 2007, 125, 111-119. https://doi.org/10.1016/j.cattod.2007.01.071
- Alvarenga, R. A. F.; Dewulf, J. Renew. Energy 2013, 59, 49-52. https://doi.org/10.1016/j.renene.2013.03.029
- Kawaguchi, H.; Ogino, C.; Kondo, A. Bioresour. Technol. 2017, 245, 1664-1673. https://doi.org/10.1016/j.biortech.2017.06.135
- Berti, C.; Binassi, E.; Colonna, M.; Fiorini, M.; Kannan, G.; Karanam, S.; Mazzacurati, M.; Odeh, I. US Patent 2,010,168,371, 2010
- Sousa, A. F.; Patricio, R.; Terzopoulou, Z.; Bikiaris, D. N.; Stern, T.; Wenger, J.; Loos, K.; Lotti, N.; Siracusa, V.; Szymczyk, A.; Paszkiewicz, S.; Triantafyllidis, K. S.; Zamboulis, A.; Nikolic, M. S.; Spasojevic, P.; Thiyagarajan, S.; van Es D. S.; Guigom, N. Green Chem. 2021, 23, 8795-8820.
- Zhang, D.; Dumont, M. J. J. Polym. Sci. A Polym. Chem. 2017, 5(9), 1478-1492. https://doi.org/10.1002/pola.28527
- Niaounakis, M. Biopolymers: Applications and Trends, William Andrew/Elsevier, Oxford, UK, 2015.
- Siracusa, V.; Blanco, I. Polymers 2020, 12 (8), 1641 https://doi.org/10.3390/polym12081641
- http://www.pepsico.co.uk, accessed 2022
- Sharon, C.; Sharon, M. J. Microbiol. Biotechnol. Res. 2012, 2 (2), 248-257.

This work is licensed under a Creative Commons Attribution 4.0 International License.









