Zinc Nanomaterials: A Double-Edged Sword in Detection and Prevention Against SARS-Cov-2 Spread
Mamta, Subhash and Ashu Chaudhary*
Department of chemistry, Kurukshetra University, Kurukshetra-136119, Haryana, India.
Corresponding Author E-mail: ashuchaudhary21@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380314
Article Received on : 24 Feb 2022
Article Accepted on : 27 Jun 2022
Article Published : 28 Jun 2022
Reviewed by: Dr. A. Nemeryuk
Second Review by: Dr. Vijay Bharti
Final Approval by: Dr. Mohsen Mhadhbi
The rise of ongoing Covid SARS-CoV-2 drove pandemic disease has created the perpetual interest for the assessment and improvement of reasonable progressed materials for controlling this and future unexpected viral diseases. One of the fundamental worries about this pandemic situation is the analysis and identification of infected patients. In this regard, the utilization of zinc-based nanomaterials to identify the vital biological markers of the SARS-CoV-2 remains a prevalent bother, whereas the advancement of particular and delicate devices is the essential objective. To obstruct virus proliferation, the expanding interest for self-disinfected covering requires elective materials to satisfy this problem. In this unique situation, zinc nanomaterials have given a fundamental commitment to the administration of Covid-19. Zinc nanomaterials have displayed huge antiviral action against a few infections like flu and Covids. This review delineates the importance of nanotechnology mediation in settling this tough condition.
KEYWORDS:Antiviral; Disinfectant; Nanomaterials; Nanobiosensors; SARS-CoV-2
Download this article as:| Copy the following to cite this article: Mamta M, Subhash S, Chaudhary A. Zinc Nanomaterials: A Double-Edged Sword in Detection and Prevention Against SARS-Cov-2 Spread. Orient J Chem 2022;38(3). |
| Copy the following to cite this URL: Mamta M, Subhash S, Chaudhary A. Zinc Nanomaterials: A Double-Edged Sword in Detection and Prevention Against SARS-Cov-2 Spread. Orient J Chem 2022;38(3).Available from: https://bit.ly/3yqnLbY |
Introduction
Respiratory viruses address an extreme general wellbeing hazard around the world, and the research commitment to handle the ongoing epidemic caused by the severe acute respiratory syndrome coronavirus 2 is the fundamental focuses among the various scientific organizations. To tackle this issue, specialists from various fields have accumulated to defy the devastating epidemic 1. Up to this point, broad procedures have been executed for the quick determination, anticipation, control, and treatment of the SARS-CoV-2 infection 2. Because of the great contagiousness rate and the need for sufficient personal protective equipment, the severe acute respiratory syndrome coronavirus 2 pandemic addresses a significant test to the medicinal industry 3-4. In such a manner, nanotechnology has been utilized to manage the SARS-CoV-2 and other infections. Nanotechnology is a type of biomimetic innovation. Viruses are nanoparticle-sized. Therefore, fundamental knowledge of nanotechnology is crucial. Nanotechnology can be comprehensively characterized as the plan and utilization of a few materials and devices of at least one dimension should be under 100 nm 9-12. Throughout the long term, nanoparticles have been widely utilized and concentrated because of their extraordinary properties, like small size, further developed dissolvability, surface versatility, and multifunctionality, bringing about the improvement of better and more secure medications, tissue-designated medicines, customized nanomedicines, and early conclusion and avoidance of diseases 13-14. In this way, it appears to be that nano-based methodologies soon will be the best option for the advancement of the best treatments for a wide scope of diseases. Almost certainly, nanotechnology composes immense capability in the finding, medication, and avoidance of coronavirus disease 2019. Metal and its oxide nanoparticles have been helpful in such a manner. These have been broadly concentrated because of their outstanding and novel characteristics 15,16. Various utilization of these materials has been accounted for in different industries, including medication and drug stores. Metallic, metal oxide, or other nanoparticles like gold, silver, copper, copper (II) oxide, and zinc (II) oxide, can decrease chances connected with the Covid-19 pandemic 17-20. Compared with other metal oxide nanoparticles, zinc oxide nanoparticles definitely stand out because of their benefit of having great biocompatibility with human cells. Zinc oxide nanoparticles have direct antiviral action against numerous viruses. Zinc has antiviral activity as it can avert viral entry, viral replication, and spreading to organs and in the end can set off reactive oxygen species (ROS) prompting oxidative injury and viral demise 21,22. In the course of a viral infection, Zn has been discovered to increase both innate and adaptive immunity. In addition, Zn supplementation (using various nano Zn compounds) can help SARS-CoV-2 patients recover faster 23-27. The main worry about the severe acute respiratory syndrome coronavirus 2 is its high infectiousness, expanding the death rate around the world. The fundamental transference, for this situation, is through removed drops from infected people or in a roundabout way by contact with polluted surfaces. Because of these reasons, it becomes crucial to recognize the infected people in the beginning phases of the disease and furthermore the advancement of disinfectant against SARS-CoV-2 infection. In such a manner, zinc-based nanomaterials assume a huge part (Figure 1.) 23-26. Thus, because of the capability of zinc oxide nanoparticles to control the pandemic of infection around the world, it is important to gather the most recent techniques and new accomplishments in such a manner and make them accessible to intrigued researchers.
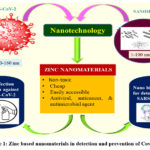 |
Figure 1: Zinc based nanomaterials in detection and prevention of Covid-19. |
Zinc Nanoparticles: A Promising Antiviral Agent
This new Covid pandemic has shown promising outcomes, looking for an integral treatment to help avoidance, treatment, and retrieval. In this specific situation, a few researches have explored the functionalities of metal nanoparticles to expand their specificity and viability as opposed to respiratory infections because these nanoparticles can tie with the proteins generated by a virus and can assist with conducing its inactivation 27,28. Likewise, in twofold stranded ribonucleic acid viruses, silver nanoparticles have revealed that after binding with the viral genome, they hinder viral reproduction; similarly, copper nanoparticles restrain virus-cell interaction and connection and obliterate the viral genome, though iron nanoparticles tie to the virus to avert it from binding to a living cell that serves as a shelter and a food source for this virus. However, selenium nanoparticles shield from apoptosis brought about by the infection of the virus 29. On account of zinc nanoparticles, it has been seen that it disrupts viral deoxyribonucleic acid (DNA) polymerase movement and ties to virions bringing about restraint in viral replication and entry 30. In spite of the fact that the specific action of zinc nanomaterials to go about as specialists for the cure of the intermittent respiratory infection is as yet being scrutinized, cell film nanoparticle electrostatic collaboration, ROS generation, and intracellular Zn2+ discharge are relied upon to be liable for diminishing cell feasibility 31-37. Also, docking molecular investigations have been executed to decide the association between zinc oxide nanoparticles and the normal severe acute respiratory syndrome coronavirus 2 targets. An investigation hypothesized the conceivable cooperation between zinc oxide nanoparticles and ACE2 receptor, SARS-CoV-2 RNA-dependent RNA polymerase (RdRp), and the primary protease, advancing the consideration towards the interaction of zinc oxide nanoparticles with the tried targets and noticing an improved dose-dependent cell take-up 38. It was additionally observed that zinc oxide could impersonate the hindrance conduct of GS-5734 and Invirase medicines by H-bond interactions with the Arg555, Ser759 amino acids, and U10, U20 uracil bases, respectively 39. Antiviral activity of Zn NPs further relies upon morphological parts of nanoparticles, for example, molecule size, shape, fixation, aggregation, colloidal detailing, and media pH 27-28. In this unique circumstance, PEGylated zinc oxide nanoparticles have preferable antiviral action over uncovered zinc oxide (ZnO) nanoparticles, essentially diminishing H1N1 infection titer post-disease 38,40,41. Similarly, the antiviral action of PEGylated zinc oxide nanoparticles is dose subordinate compared with an antiviral medication, seeing the best H1N1 antiviral impact with a restraint rate 41. In antiviral treatment, zinc oxide nanoparticles offer benefits and efforts to challenge the event of drug resistance. The consequences of Zn-based nanomaterials for antiviral action against the SARS-CoV-2 are summarized in Table 1 37, 38, 42, 43. The genuine pandemic circumstance has underscored the critical requirement for new antiviral remedial. From the above conversation, it tends to be seen that extensive attempts have been directed on creating zinc nanoparticles, and thus, these materials have shown significant functionalities as prophylactic agents, acting against viral deoxyribonucleic acid polymerase action and viral reproduction, assisting with restricting the disease obstruction handled by virus mutations and binding to target proteins to diminish entanglements 44. Consequently, scientific proof acquired through extra research can add significant establishments to cure a viral infection.
Table 1: Antiviral activity by zinc based nanomaterials against SARS-CoV-2
|
Zn Nanomaterials |
Cells/study |
Effects |
Comments |
Ref |
|
ZnO NPs (Nano-Spray) |
VeroE6 cells, antiviral activity |
ZnO NPs inhibit the virus activation by zinc (II) release and ROS formation |
Potent antiviral activity at a very low concentration |
[37] |
|
ZnO NPs |
severe acute respiratory syndrome coronavirus 2 targets RNA dependent RNA polymerase and ACE2 receptor in human lung fibroblast cells |
The binding of zinc oxide nanoparticles with the inspected coronavirus diseases 2019 targets through H- bond formation was found. |
Interpret more biological and helpful investigations. |
[38] |
|
ZnO/ BER complex |
VeroE6 cells, anti-Covid activity |
This complex hinders the virus entry through cooperating with the ACE2 enzyme on the cell surface, the spike protein interaction, PLpro activity and reduces the expression of RdRp, |
A significant anti-Covid agent because it inhibited the virus entry, replication, and assembly. |
[42] |
|
ZnO NFs (Nano Flowers) |
SARS-CoV-2 spike protein and ACE2 receptor in human lung epithelial cells |
Significant denaturation of the spike proteins on the zinc oxide surface revealing removal of the virus upon efficient trapping |
Attachment of ZnO NFs to cotton develops the nanoceutical fiber which can be used as anti- CoV-2 washable dress material. |
[43] |
Implementation Of Zinc Based Nanomaterials In Fighting Against Sars-Cov-2
Zinc Based Nanobiosensing Scaffolds for Detection of SARS-CoV-2
Viruses are an ordinary illusion of intermittent nanostructured materials in nature; for example, on account of the severe acute respiratory syndrome coronavirus 2, it is normally portrayed as a round shape molecule whose width is from 100 to 120 nm [50]. Thus, the discovery of little biological markers has been improved by executing nanomaterials into numerous biosensing stages. Fostering a biosensing stage to handle the current worldwide wellbeing crisis in the vicinity of accessible nanomaterials and various bio-selective methodologies remains a prevalent task (Figure 2).
 |
Table 1: Figure 2: The working of a biosensor for the detection of SARS-CoV-2 [51] Click here to View figure |
For biosensing, a few stages depend on gold, selenium, iron, Graphene, and zinc 46-52. In any case, only the elements which have been read up for biosensor stages is zinc because of its superb optical and electronic functionalities, with the extra benefit of being modest and plentiful in features. A diversity of zinc-based nanomaterials keeps on being widely explored for recognizing respiratory infections. Zinc oxide nanomaterials have been utilized for the specific discovery of other arising infections like avian flu 53-59. In the space of discovery of respiratory infections, the creation of an immunosensor in light of designed zinc oxide nanorods (NRs) networks for flu (H1N1 SIV) discovery has been accounted for 53. Although an Au cathode was utilized in the gadget, ZnO assumed an essential part in the immobilization interaction of the caught immune response. Besides, an electrochemical sensor stage for finding highly pathogenic Asian Avian Influenza A in view of graphene/ZnO nanocomposites additionally has been accounted for 55. This work has a fascinating methodology, as the outcomes got from the amperometric study were contrasted and the proficiency of a customary agarose gel electrophoresis, at last proposing a vigorous approval for the utilization of these sorts of biosensors. A nano-flow immunosensor in view of zinc oxide nanorods become on a PDMS sensor for Avain flu infection determination utilizing an electrochemical technique has been accounted for 56. The zinc oxide nanorods permitted the immobilization of various antibodies, as well as expansion inhumanity. In the improvement of fluorescent quaternary/ternary quantum dots and AuNPs for the recognition of flu infection, ZnO employs as a precursor 57. From these works, a review is opened with regards to zinc nanomaterials and their likely use in coronaviruses diseases 2019 recognition. These encouraging devices could foster preliminaries to include explicit antibodies as opposed to the notable spike protein S1. A practical way to deal with upgrading the biosensing execution of paper-based electrochemical impedance sensing nano biosensors with working electrodes improved with upward developed zinc oxide nanowires has been accounted for 58. These nanobiosensors can separate the centralizations of IgG neutralizer (CR3022) to coronavirus 2019 in human serum tests. As examined, to recognize various respiratory infections, zinc presents a broad scope of qualities in the different stages. At times, as a piece of a nanostructured or in different conditions, it is even utilized in the association of functional groups to identify biological molecules. Consequently, it is feasible to exploit signal transduction techniques to foster various sorts of optical or electrochemical biosensors. The advancement of immunosensors depends on the explicitness of the antigen-immunizer reaction. Coronavirus infection is accounted for to incite intense antibody (IgM, IgG) reaction, and the levels of antiviral antibodies are leveled inside six days after seroconversion 60. One of the principal references for coronavirus disease 2019 location is the spike protein. This could work as a sign of the infection even in the incubation time frame and for symptomless conditions 61-63. By the way, it is proposed that other film or layer proteins can be utilized to distinguish various SARS-CoV-2 variations. This could permit the creation of extra specific biosensors in view of the information, response signals, and described the conduct of devices in light of nano zinc and related compounds. The expanding information in this wellbeing crisis proposes the examination of further developed sensor surfaces from various nanomaterials, for example, zinc nanomaterials to accomplish quick location response and high selectivity and sensitivity.
 |
Figure 3: Zinc-based nanomaterial affinity towards detection of SARS-CoV-2. |
Currently acquired data with zinc-based biosensing scaffolds for other respiratory infections could be excess and could go about as a kind of perspective manual for planning new powerful biosensors for preliminary identification of diseases. As indicated by the data sets counseled, there is a finite number of works connected with the discovery of coronavirus diseases 2019 with zinc nanomaterials, which provide the perspective for future exploration and studies.
Zinc Nanomaterials in Disinfection Protocol against SARS-Cov-2
Viruses are contagious specialists of little aspects formed of two major parts: the hereditary material, deoxyribonucleic acid or ribonucleic acid, single-stranded or twofold stranded, and a capsid, which secures and exemplifies the genome 64. A few viruses have an envelope framed by a twofold layer of glycoproteins and lipids called pericapsid or peplos. Non-encompassed viruses are extra impervious to high temperatures, acids, sanitizers, and drying, while wrapped viruses endure longer in a muggy climate but are less resistant. Infection SARS-CoV-2 is an encompassed infection portrayed by four unique primary proteins: nucleocapsid (N), spike (S), layer (M), and envelope (E) (Figure 4).
 |
Figure 4: Structure of SARS-CoV-2. |
The spike, envelope, and layer proteins fabricate the viral envelope, while nucleocapsid protein holds the RNA genome 65. SARS-CoV-2 transmission is for the most part connected with the spike protein that advances the infection staying on various surfaces. In like manner, public space offices like medical clinics, libraries, and transports are additionally prescribed to be disinfected consistently as the aberrant infectious by fomites could prompt a viral infection locally 66. Consequently, the improvement of viable viral sanitizer materials is critical, and a correlative methodology that can handle the current epidemic and potential flare-ups of arising infections is of exorbitant interest. Synthetic sanitizers active against an enormous range of microbial specialists, among that encompassed viruses, have been successfully utilized for sanitization and disinfection of surfaces and personal protective equipment 67. Notwithstanding the all-around exhibited viability of substance sanitizers, they are regularly connected with a few detriments, for example, high concentration prerequisites to acquire hundred percent viral restraint, time depending adequacy, and expected dangers to human wellbeing and climate [68]. The advancement of sterilization moves toward that can stay overlong on surfaces, start impervious to washing and erosion, with great security imperatives is a bother. Another viewpoint is presented by nanotechnology, self-cleaning surfaces, and light-determined self-sterilizations. Metallic antiviral nanoparticles are the most detailed and examined frameworks because of their innate wide scope of antimicrobial actions and adequacy at a much lower dose than their bulk partners [10]. In any case, zinc nanomaterials have shown to be effective antimicrobial materials that provide assorted significant photocatalytic, surface, and morphological functionalities to prevent and inhibit the activity of microbes. Particularly, zinc oxide nanostructures show controllable antibacterial, antifungal, and antiviral limits. In this unique situation, a sanitizer nano-spray for the arising Covid (SARS-CoV-2) in light of zinc (II) oxide nanoparticles has been accounted for. ZnO-NPs have a powerful antiviral action against SARS-CoV-2 at an exceptionally low fixation (IC50 = 526 ng/mL) and can set off many free radicals that might make oxidative pressure to SARS-CoV-2 and incite serious harm to its cell films, which is a vital outcome in battling against SARS-CoV-2 37. ZnO NPs have shown elevated efficiency against a broad diversity of Gram-positive, Gram-negative microbes, and fungi 69-72. The antimicrobial and antifungal action is firmly impacted by boundaries, for example, the nanostructures fixation, size, morphology, and surface functionalization 26, 32, which can be exactly managed with the various affidavit strategies (Figure 5).
 |
Figure 5. Disinfection mechanism of ZnO nanoparticles [78]. |
In this unique circumstance, the impact of various morphologies of ZnO nanostructures over the antimicrobial and enzyme deactivation movement has been depicted. A biomimetic strategy of pyramidal nanoparticles to function as opposed to super-resistant microbes (methicillin-resistant S. aureus), which is introduced in many clinics has been accounted for 73. One another work contrasting the antibacterial action of various morphologies of zinc oxide nanomaterials has been accounted for 74. For this situation, an upgrade in the microbial restraint of Escherichia coli and Staphylococcus aureus by zinc oxide NTs is in contrast with the one shown by commercial zinc oxide nanoparticles. The zinc oxide nanotubes morphology helps with an expansion in the surface region have been noticed and furthermore diminish the conglomeration in the framework, in this way inclining toward antibacterial action. Subsequently, Nanomaterials would significantly advance the improvement of sanitizer against SARS-CoV-2, and ZnO nanoparticles are relied upon to make additional interesting commitments in these fields.
Cytotoxicity of Zinc Oxide Nanoparticles
ZnO-NPs are one of the most widely used nanomaterials today, with applications in nearly every field. Because of their effects on the environment and human health, ZnO-NPs’ cytotoxicity is also a cause for worry. ZnO-NPs antimicrobial activity is primarily due to a series of processes that occur when ZnO-NPs are activated by light. Hydrogen peroxide (H2O2) and other intermediate reactive oxygen species are produced as a result of these processes. The buildup of zinc oxide nanoparticles in bacterial cells, as well as the creation of reactive oxygen species in the cell’s internal and exterior environments, causes damage to many of the cell’s structures and, as a result, cell death 81-83. The process of ROS formation for cellular oxidative stress can be used to measure the level of toxicity of NPs 84. The cytotoxicity of bare zinc oxide nanoparticles on HepG2 cells studied by Elje et al. and discovered that cytotoxicity is dose dependent 85. Jevapatarakul et al. produced zinc oxide nanoparticles using a green approach and tested their cytotoxicity on skin cancer cells (A431). They also came to the conclusion that zinc oxide nanoparticles cytotoxicity is dose and concentration dependent [86]. The cytotoxicity of zinc oxide nanoparticles on MCF-7 cells studied by Khorsandi et al. and they reported the same observations as in the earlier references 87. On HepG2 cells, Chen et al. investigated the cytotoxicity of bare zinc oxide nanoparticles. They came to the conclusion that cytotoxicity is dependent on NP size and concentration 88. The cytotoxicity of metal oxide NPs coated with EDTMP was investigated by Xiaoming et al. They determined that metal oxide can cause the production of reactive oxygen species (ROS) and cell death as a result of oxidative stress but that the surface activity of metal oxide is reduced by the coating layer. THP-1 and BEAS-2B cells revealed low ROS production and oxidative damage when exposed to EDTMP coated metal oxide 89. The cytotoxicity of zinc oxide and copper oxide nanoparticles coated with polyethylene glycol and polyvinyl alcohol was also studied by Negvenkar et al. They discovered that polymer encapsulation of NPs reduced cytotoxicity toward bacteria 91. The same findings were reported by Luisa et al., who found that coating of zinc oxide nanoparticles with polyethylene glycol and polyethylenimine lowers their cytotoxicity on lung cells [91]. Khalid et al. also reported the cytotoxicity of ZnO-NPs coated with folic acid and polyethylene glycol. They found that folic acid and polyethylene glycol capped ZnO NPs showed a reduction in cytotoxicity as compared to individual zinc oxide nanoparticles 92.
Conclusion
With the epidemic brought about by coronavirus diseases 2019, it is important to look for procedures to contain this novel and dangerous human disease. In this regard, Nanotechnology has effectively been displayed to upgrade diagnosis, security, and treatments in other viral infections; hence, there is a decent opportunity that, with more innovative work, it will reform the battle against coronavirus diseases 2019, presenting processes, materials, and apparatuses to improve awareness, speed, and authenticity of detection, as well as giving more adequate choices to treatments. From the above studies, it is concluded that zinc-based nanomaterials have intense antiviral action. There is a finite number of works studied on zinc-based nanomaterials utilized in disinfectant and diagnosis of COVID-19 virus, which provide the perspective for future exploration and studies. It is also concluded that the cytotoxicity of zinc oxide nanoparticles can be reduced by coating them with suitable polymers.
Acknowledgement
The authors Mamta and Subhash are thankful to the UGC (NTA Ref. No. 191620133605, CSIR-UGC NET DEC. 2019 and Ref. No. 92, CSIR-UGC NET DEC. 2018), New Delhi, India respectively for financial assistance in the form of JRF.
Conflict of Interest
The authors declare that they have no conflict of interest regarding the publication of this article.
Funding Sources
There is no funding sources.
References
- Rodelo, C.G.; Salinas, R.A.; Jaime, E.A.; Armenta, S.; Galdámez-Martínez, A.; Castillo-Blum, S.E.; Astudillo-de la Vega, H.; Grace, A.N.; Aguilar-Salinas, C.A.; Rodelo, J.G.; Christie, G.; Alsanie, W.F.; Santana, G.; Thakur, V.K.; Dutt, A.; Rodelo C.G.; Salinas, R.A.; Jaime, E.A.; Armenta, S.; Galdámez-Martínez, A. Coordination Chemistry Reviews 2022, 457, 214402.
- Shadpour, M.; Elham, A.; Chaudhery, M. H. New J. Chem. 2021, 45, 6167–6179.
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; Wang, X. Nature 2020, 581, 215–220.
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; Chen, H.-D.; Chen, J.; Luo, Y.; Guo, H.; Jiang, R.-D.; Liu, M.-Q.; Chen, Y.; Shen, X.-R.; Wang, X.; Zheng, X.-S.; Zhao, K.; Chen, Q.-J.; Deng, F.; Liu, L.-L.; Yan, B.; Zhan, F.-X.; Wang, Y.-Y.; Xiao, G.-F.; Shi, Z.-L. Nature 2020, 579, 270−273.
- Gil, C.; Ginex, T.; Maestro, I.; Nozal, V.; Barrado-Gil, L.; Cuesta- Geijo, M. Á.; Urquiza, J.; Ramírez, D.; Alonso, C.; Campillo, N. E.; Martinez, A. J. Med. Chem. 2020, 63, 12359-12386.
- Cui, J.; Li, F.; Shi, Z.-L. Nat Rev Microbiol. 2019, 17, 181–192.
- Letko, M.; Marzi, A.; Munster, V. Nat Microbiol. 2020, 5, 562–569.
- Jin, Y.-H.; Cai, L.; Cheng, Z.-S.; Cheng, H.; Deng, T.; Fan, Y.-P.; Fang, C.; Huang, D.; Huang, L.-Q.; Huang, Q.; Han, Y.; Hu. B.; Hu, F.; Li, B.-H.; Li, Y.-R.; Liang, K.; Lin, L.-K.; Luo, L.-S.; Ma, J.; Ma, L.-L.; Peng, Z.-Y.; Pan, Y.-B.; Pan, Z.-Y.; Ren, X.-Q.; Sun, H.M.; Wang, Y.; Wang, Y.-Y.; Weng, H.; Wei, C.-J.; Wu, D.-F.; Xia, J.; Xiong, Y.; Xu, H.-B.; Yao, X.-M.; Yuan, Y.F.; Ye, T.S.; Zhang, X.C.; Zhang, Y.W.; Zhang Y.-G.; Zhang, H.M.; Zhao, Y.; Zhao, M.-J.; Zi, H.; Zeng, X.-T.; Wang, Y.-Y.; Wang, X.-H. Military Med Res. 2020, 7, 4.
- Bhavana, V.; Thakor, P.; Singh, S. B.; Mehra, N. K. Life Sci. 2020, 261, 118336.
- Talebian, S.; Wallace, G.G.; Schroeder, A.; Stellacci, F.; Conde, J. Nat. Nanotechnol. 2020, 15, 618–621.
- Campos, E.V.R.; Pereira, A.E.S.; De Oliveira, J.L.; Carvalho, L.B.; Guilger- Casagrande, M.; De Lima, R.; Fraceto, L.F. J. Nanobiotechnol. 2020, 18, 1–23.
- Mokhtarzadeh, A.; Eivazzadeh-Keihan, R.; Pashazadeh, P.; Hejazi, M.; Gharaatifar, N.; Hasanzadeh, M.; Trends Anal Chem. 2017, 97, 45–57.
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Front Chem. 2018, 6, 360.
- Fornaguera, C.; García-Celma, M.J. J Personal Med. 2017, 7, 12–20.
- Zhang, D.; Yang, Z.; Yu, S.; Mi, Q.; Pan, Q. Coord. Chem. Rev. 2020, 413, 213272.
- Reddy, R.C.K.; Lin, J.; Chen, Y.; Zeng, C.; Lin, X.; Cai, Y.; Su, C.Y. Coord. Chem. Rev. 2020, 420, 213434.
- Du, T.; Zhang, J.; Li, C.; Song, T.; Li, P.; Liu, J.; Du, X.; Wang, S. Bioconjugate Chem. 2020, 31, 2553–2563.
- Behzadinasab, S.; Chin, A.; Hosseini, M.; Poon, L.; Ducker, W.A. ACS Appl. Mater. Interfaces 2020, 12, 34723–34727.
- Chaco´n-Torres, J.C.; Reinoso, C.; Navas-Leo´n, D.J.; Bricen˜o, S.; Gonza´lez, G. Sci. Rep. 2020, 10, 19004.
- Raval, N.P.; Kumar, M. J. Hazard. Mater. 2021, 402, 123466.
- Tsuneo, I. Curr. Trends Biomed. Eng. Biosci. 2019, 19, 1–6.
- Kalyani, R.L.; Pammi, S.V.N.; Vijay Kumar, P.P.N.; Swamy, P.V.; Ramana Murthy, K.V. Mater. Sci. Eng. C 2019, 103, 109756.
- Rahman, M.T.; Idid, S.Z. Biol. Trace Elem. Res. 2021, 199, 550-558.
- Kumar, A.; Kubota, Y.; Chernov, M.; Kasuya, H. Med. Hypotheses. 2020, 144, 109848.
- Razzaque, M.S.; Tohoku J. Exp. Med. 2020, 251, 175-181.
- Carlucci, P.M.; Ahuja, T.; Petrilli, C.; Rajagopalan, H.; Jones, S.; Rahimian, J. J. Med. Microbiol. 2020, 69, 1228-1234.
- Derwand, R.; Scholz, M. Med. Hypotheses. 2020, 142, 109815.
- Gorshkov, K.; Susumu, K.; Chen, J.; Xu, M.; Pradhan, M.; Zhu, W.; Hu, X.; Breger, J.C.; Wolak, M.; Oh, E. ACS Nano 2020, 14, 12234–12247.
- Espitia, P.J.P.; Soares, N.F.F.; Coimbra, J.S.R.; De Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Food Bioprocess Technol. 2012, 5, 1447–1464.
- Pasquet, J.; Chevalier, Y.; Pelletier, J.; Couval, E.; Bouvier, D.; Bolzinger, M.A. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 457, 263–274,
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Nano-Micro Lett. 2015, 7, 219–242.
- Siddiqi, K.S.; ur Rahman, A.; Tajuddin, H.A. Nanoscale Res. Lett. 2018, 131, 1–13.
- Dawre, S.; Maru, S. Life Sci. 2021, 278, 119561.
- Maduray, K.; Parboosing, R. Biol. Trace Elem. Res. 2020, 199, 3159–3176.
- Attia, G.H.; Moemen, Y.S.; Youns, M.; Ibrahim, A.M.; Abdou, R.; El Raey, M.A. Colloids Surfaces B Biointerfaces 2021, 203, 111724.
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Appl. Environ. Microbiol. 2011, 77, 2325–2331.
- Sharmin, S.; Rahaman, M.M.; Sarkar, C.; Atolani, O.; Islam, M.T.; Adeyemi, O.S. Heliyon. 2021, 7, e06456.
- Jalal, R.; Goharshadi, E.K.; Abareshi, M.; Moosavi, M.; Yousefi, A.; Nancarrow, P. Mater. Chem. Phys. 2010, 121, 198–201.
- Lipovsky, A.; Nitzan, Y.; Gedanken, A.; Lubart, R. Nanotechnology 2011, 22, 105101.
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. nanomedicines nanotechnology, Biol. Med. 2011, 7, 184–192.
- Sevinç, B.A.; Hanley, L. J. Biomed. Mater. Res. – Part B Appl. Biomater. 2010, 94, 22–31.
- El-Megharbel, S.M.; Alsawat, M.; Al-Salmi, F.A.; Hamza, R.Z. Coatings. 2021, 11, 388.
- Pormohammad, A.; Monych, N.K.; Turner, R.J. Int. J. Mol. Med. 2021, 47, 326–334.
- Hamdi, M.; Abdel-Bar, H.M.; Elmowafy, E.; El-Khouly, A.; Mansour, M.; Awad, G.A.S. ACS Omega 2021, 6, 6848–6860.
- Tavakoli, A.; Ataei-Pirkooh, A.; MM Sadeghi, G.; Bokharaei-Salim, F.; Sahrapour, P.; Kiani, S.J.; Moghoofei, M.; Farahmand, M.; Javanmard, D.; Monavari, S.H. Nanomedicine. 2018, 13, 2675–2690.
- Ghaffari, H.; Tavakoli, A.; Moradi, A.; Tabarraei, A.; Bokharaei-Salim, F.; Zahmatkeshan, M.; Farahmand, M.; Javanmard, D.; Kiani, S.J.; Esghaei, M.; Pirhajati-Mahabadi, V.; Ataei-Pirkooh, A.; Monavari, S.H. J. Biomed. Sci. 2019, 26, 70.
- Ghareeb, D.A.; Saleh, S.R.; Seadawy, M.G.; Nofal, M.S.; Abdulmalek, S.A.; Hassan, S.F.; Khedr, S.M.; AbdElwahab, M.G.; Sobhy, A.A.; Saber, A.; Abdel-Hamid, A.; Yassin, A.M.; Elmoneam, A.A.A.; Masoud, A.A.; Kaddah, M.M.Y.; El-Zahaby, S.A.; Al-mahallawi, A.M.; El-Gharbawy, A.M.; Zaki, A.; Seif, I.K.; Kenawy, M.Y.; Amin, M.; Amer, K.; El Demellawy, M.A. J. Pharm. Investig. 2021, 51, 1-23.
- Adhikari, A.; Pal, U.; Bayan, S.; Mondal, S.; Ghosh, R.; Darbar, S.; Saha-Dasgupta, T.; Ray, S.K.; Pal, S.K. ACS Appl. Bio Mater. 2021, 4, 5471–5484.
- Mcpherson, S.W.; Keunen, J.E.; Bird, A.C.; Chew, E.Y.; Van Kuijk, F.J. American journal of ophthalmology 2020, 216, A5-A6.
- Sharifi, M.; Hasan, A.; Haghighat, S.; Taghizadeh, A.; Attar, F.; Bloukh, S.H.; Edis, Z.; Xue, M.; Khan, S.; Falahati, M. Talanta 2021, 223, 121704.
- Pradhan, A.; Lahare, P.; Sinha, P.; Singh, N.; Gupta, B.; Kuca, K.; Ghosh, K.K.; Krejcar, O. Sensors 2021, 21, 7823.
- Moitra, P.; Alafeef, M.; Dighe, K.; Frieman, M.B.; Pan, D. ACS Nano 2020, 14, 7617–7627,
- Alafeef, M.; Dighe, K.; Moitra, P.; Pan, D. ACS Nano 2020, 14, 17028–17045.
- Qiu, G.; Gai, Z.; Tao, Y.; Schmitt, J.; Kullak-Ublick, G.A.; Wang, J. ACS Nano 2020, 14, 5268–5277.
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.H.; Choi, M.; Ku, K.B.; Lee, C.S.; Jun, S.; Park, D.; Kim, H.G.; Kim, S.J.; Lee, J.O.; Kim, B.T.; Park, E.C.; Kim, S.I. ACS Nano 2020, 14, 5135–5142.
- Ali, M.A.; Hu, C.; Jahan, S.; Yuan, B.; Saleh, M.S.; Ju, E.; Gao, S.J.; Panat, R. Adv. Mater. 2020, 32, 1–15.
- Roh, C.; Jo, S.K. J. Chem. Technol. Biotechnol. 2011, 86, 1475–1479.
- Tian, B.; Gao, F.; Fock, J.; Dufva, M.; Hansen, M.F. Biosens. Bioelectron. 2020, 165, 112356
- Jang, Y.; Park, J.; Pak, Y.K.; Pak, J.J. J. Nanosci. Nanotechnol. 2012, 12, 5173–5177.
- Nguyen, A.V.T.; Dao, T.D.; Trinh, T.T.T.; Choi, D.Y.; Yu, S.T.; Park, H.; Yeo, S.J. Biosens. Bioelectron. 2020, 155, 112090.
- Low, S.S.; Tan, M.T.T.; Loh, H.S.; Khiew, P.S.; Chiu, W.S. Anal. Chim. Acta. 2016, 903, 131–141.
- Han, J.H.; Lee, D.; Chew, C.H.C.; Kim, T.; Pak, J.J. Sens. Actuators, B Chem. 2016, 228, 36–42.
- Nasrin, F.; Chowdhury, A.D.; Takemura, K.; Kozaki, I.; Honda, H.; Adegoke, O.; Park, E.Y. Anal. Chim. Acta 2020, 1109, 148–157.
- Li, X.; Qin, Z.; Fu, H.; Li, T.; Peng, R.; Li, Z.; Rini, J.M.; Liu, X. Biosens. Bioelectron. 2021, 177, 112672.
- Xiao, M.; Huang, L.; Dong, X.; Xie, K.; Shen, H.; Huang, C.; Xiao, W.; Jin, M.; Tang, Y. Analyst 2019, 144, 2594–2603.
- Long, Q.X.; Liu, B.Z.; Deng, H.J.; Wu, G.C.; Deng, K.; Chen, Y.K.; Liao, P.; Qiu, J.F.; Lin, Y.; Cai, X.F.; Wang, D.Q.; Hu, Y.; Ren, J.H.; Tang, N.; Xu, Y.Y.; Yu, L.H.; Mo, Z.; Gong, F.; Zhang, X.L.; Tian, W.G.; Hu, L.; Zhang, X.X.; Xiang, J.L.; Du, H.X.; Liu, H.W.; Lang, C.H.; Luo, X.H.; Wu, S.B.; Cui, X.P.; Zhou, Z.; Zhu, M.M.; Wang, J.; Xue, C.J.; Li, X.F.; Wang, L.; Li, Z.J.; Wang, K.; Niu, C.C.; Yang, Q.J.; Tang, X.J.; Zhang, Y.; Liu, X.M.; Li, J.J.; Zhang, D.C.; Zhang, F.; Liu, P.; Yuan, J.; Li, Q.; Hu, J.L.; Chen, J. Nat. Med. 2020, 26, 845–848.
- Tan, Y.J.; Goh, P.Y.; Fielding, B.C.; Shen, S.; Chou, C.F.; Fu, J.L.; Leong, H.N.; Leo, Y.S.; Ooi, E.E.; Ling, A.E.; Lim, S.G.; Hong, W. Clin. Diagn. Lab. Immunol. 2004, 11, 362–371.
- Lu, L.; Manopo, I.; Leung, B.P.; Chng, H.H.; Ling, A.E.; Chee, L.L.; Ooi, E.E.; Chan, S.W.; Kwang, J. J. Clin. Microbiol. 2004, 42, 1570–1576.
- Qiu, M.; Shi, Y.; Guo, Z.; Chen, Z.; He, R.; Chen, R.; Zhou, D.; Dai, E.; Wang, X.; Si, B.; Song, Y.; Li, J.; Yang, L.; Wang, J.; Wang, H.; Pang, X.; Zhai, J.; Du, Z.; Liu, Y.; Zhang, Y.; Li, L.; Wang, J.; Sun, B.; Yang, R. Microbes Infect. 2005, 7, 882–889.
- Golin, A.P.; Choi, D.; Ghahary, A. Am. J. Infect. Contr. 2020, 48, 1062–1067.
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; Zheng, M.; Chen, L.; Li, H. .Acta Pharm. Sin. B. 2020, 10, 766–788.
- Rakowska, P.D.; Tiddia, M.; Faruqui, N.; Bankier, C.; Pei, Y.; Pollard, A.J.; Zhang, J.; Gilmore, I.S. Commun. Mater. 2021, 2, 53.
- Fathizadeh, H.; Maroufi, P.; Momen-Heravi, M.; Dao, S.; Köse, S.; Ganbarov, K.; Pagliano, P.; Esposito, S.; Kafil, H.S. Inf. Med. 2020, 28, 185–191.
- Wang, J.; Shen, J.; Ye, D.; Yan, X.; Zhang, Y.; Yang, W.; Li, X.; Wang, J.; Zhang, L.; Pan, L. Environ. Pollut. 2020, 262, 114665.
- Shaban, M.; Mohamed, F.; Abdallah, S. Sci. Rep. 2018, 8, 1–15.
- Janaki, A.C.; Sailatha, E.; Gunasekaran, S. Spectrochim. Acta – Part A Mol. Biomol. Spectrosc. 2015, 144, 17–22
- De Souza, R.C.; Haberbeck, L.U.; Riella, H.G.; Ribeiro, D.H.B.; Carciofi, B.A.M. Brazilian J Chem. Eng. 2019, 36, 885–893.
- Dimapilis, E.A.S.; Hsu, C.S.; Mendoza, R.M.O.; Lu, M.C. Sustain. Environ. Res. 2018, 28, 47–56.
- Cha, S.H.; Hong, J.; McGuffie, M.; Yeom, B.; Vanepps, J.S.; Kotov, N.A. ACS Nano 2015, 9, 9097–9105.
- De Dicastillo, C.L.; Vidal, C.P.; Falcó, I.; Sánchez, G.; Márquez, P.; Escrig, J. Nanomaterials 2020, 10, 1–14.
- Król, A.; Pomastowski, P.; Rafinska, K.; Railean-Plugaru, V.; Buszewski, B. Adv. Colloid Interface Sci. 2017, 249, 37–52.
- Siddiqi, K. S.; ur Rahman, A.; Husen, A. Nanoscale Res. Lett. 2018, 13, 141.
- Sirelkhatim, A., Mahmud, S., Seeni, A., Kaus, N. H. M., Ann, L. C., Bakhori, S. K. M., Hasan, H.; Mohamad, D. Nano-Micro Letters 2015, 7, 219–242.
- Kaweeteerawat, C. Environ. Sci. Technol. 2015, 49, 1105-1112.
- Elje, E.; Mariussen, E.; Moriones, O.H.; Bastús, N.G.; Puntes, V.; Kohl, Y.; Dusinska, M.; Rundén-Pran, E. Nanomaterials 2020, 10, 545.
- Jevapatarakul, D.; T-Thienprasert, J.; Payungporn, S.; Chavalit, T.; Khamwut, A.; T-Thienprasert, N.P. Biomed. Pharmacother. 2020, 130, 110552.
- Khorsandi, L.; Farasat, M.; Environ. Sci. Pollut. Res. 2020. 27, 38300.
- Chen, P., Wang, H.; He, M.; Chen, B.; Yang, B.; Hu, B. Ecotoxicol. Environ. Saf. 2019, 171, 337-346.
- Cai, X.; Lee, A.; Ji, Z.; Huang, C.; Chang, C.H.; Wang, X.; Liao, Y.P.; Xia, T.; Li, R. Part. Fibre Toxicol. 2017, 14, 13.
- Nagvenkar, A.P.; Perelshtein, I.; Piunno, Y.; Mantecca, P.; Gedanken, A. ACS omega 2019, 4, 13631.
- Fiandra, L.; Bonfanti, P.; Piunno, Y.; Nagvenkar, A.P.; Perlesthein, I.; Gedanken, A.; Saibene, M.; Colombo, A.; Mantecca, P. NanoImpact 2020, 17, 100195.
- Khalid, A.D.; ur-Rehman, N.; Hadi, F.; Iqbal, S.S.; Buzdar, S.A.; Khan, A.K. Dig. J. Nanomater. Biostructures 2022, 17, 73 – 79.

This work is licensed under a Creative Commons Attribution 4.0 International License.









