Oxide Based Nanoparticles: A review
Mukhan Wati1* , Komal Hudda2
, Komal Hudda2 , Barkha Rathee1
, Barkha Rathee1 , Sweety Ranga1
, Sweety Ranga1 , Ravinder Sindhu3
, Ravinder Sindhu3
1Department of Chemistry, Maharshi Dayanand University,Rohtak-124001, Haryana, India.
2Vaish Arya Kanya Mahavidyalaya, Bahadurgarh, Hryana, India.
3Department of Physics, Kurukshetra UniversityKurukshetra-136119, Haryana, India.
Corresponding Author E-mail: mukhandagar.rp.chem@mdurohtak.ac.in
DOI : http://dx.doi.org/10.13005/ojc/380315
Article Received on : 15-Apr-2022
Article Accepted on : 10-May-2022
Article Published : 16 Jun 2022
Reviewed by: Dr. Yunusov Khaydar Ergashovich
Second Review by: Dr. Ashish Goel
Final Approval by: Dr. Mohsen Mhadhbi
In this report we discussed about the properties of magnetic nanoparticles which are important for the synthesis of nanoparticles of particular use. Various oxide-based nanoparticles can be synthesized depending on the properties which are needed for their applications. Various methods have been presented that offer control over the size, growth of the nanoparticles. Among the methods reported, hydrothermal method probably offers the most promising method for control and scalability.
KEYWORDS:Crystal structure; Magnetic nanoparticles; Magnetic property; Techniques
Download this article as:| Copy the following to cite this article: Wati M, Hudda K, Rathee B, Ranga S, Sindhu R. Oxide Based Nanoparticles: A review. Orient J Chem 2022;38(3). |
| Copy the following to cite this URL: Wati M, Hudda K, Rathee B, Ranga S, Sindhu R. Oxide Based Nanoparticles: A review. Orient J Chem 2022;38(3).Available from: https://bit.ly/39t17Gd |
Introduction
Beginning of twentieth century uncovered the magnetic phenomenon at atomic scale but the discovery of magnetic material, iron oxide Fe3O4 reformed the field of magnetism.
These magnetic particles when broken down to nanometer scale have most promising applications because of their multiple uses. Size controlled synthesis of these nanoparticles can be done in several physical and chemical ways. The nanoscale synthesis leads to their use at the biological level of cells, proteins, genes and viruses. The optimized size of nanoparticles, their assortment, layering, redistribution of sizes, their shapes and their distinctive magnetic properties became a cause of their application in various fields. They are used in formulations of agrochemicals, electronics, biomedicine, computing, biosensors, electrodes, storage media and astrionics.1-3
Small size of these particles allows them to interact with biological system and this prompted their use in biotechnology and biomedicine. Among a variety of nanomaterials, magnetic nanomaterials play an immense role in areas such as contrast agents for MRI, biosensors, drug delivery, repairing of cells and tissues, magnetic separation and hyperthermia.
These magnetic nanoparticles have many biomedical applications and this has attracted much of our interest towards their synthesis, structure-property relationship and characterization strategies. Diverse uses of nanostructured magnetic particles have led to many attempt to prepare nanoparticles in optimized shapes and sizes. The core issue of magnetic nanoparticles is understanding their magnetic properties.
Temperature, applied magnetic field, and pressure are the factors governing the magnetic properties of the materials. Variation of the above parameters results in various magnetic forms. These particles can take up two shapes crystalline or amorphous, depending on the procedure of their formation. Monodisperse nanoparticles for uses in nanotechnology have been synthesized to a substantial extent. It is the method of synthesis which determines the presence of structural defects as well as the impurities in a particle. Number of impurities present in a particle and distribution of these defects inside the structure helps in discovering its magnetic properties. Definite size, structure and large ratio of surface/volume of the magnetic nanocrystals are responsible for considerably different and interesting properties than their corresponding bulk materials.
Magnetization in these particles is decided by relative orientations of the magnetic moments resulting from the unpaired valence electrons of the atoms. Electronic spin around the axis of atom and its motion around the orbit of electron is responsible for the magnetism. Common metals having a non-zero magnetic moment are Iron, Nickel, Manganese and Cobalt. Electric spin is responsible for net magnetic moment of these metals.4
The focus of the review is to shed some light on the exponential growth in this field and its outlook. Crystal structure, magnetic properties and designing of Oxide-based magnetic nanoparticles will be major concern. We will be exploring the parameters like size, shape, coating and crystalline structure which affect the properties of the magnetic nanoparticles. Simple oxides such as iron oxide, nickel oxide or complex oxides like cobalt ferrite, ferrihydrite, etc. will be dealt with. We will be comparing magnetic anisotropy, responsible for the magnetic behavior and particle size of these nanoparticles determined from XRD, TEM etc.
Magnetic Nanoparticles
Magnetic nanoparticles are a class of engineered particulate materials having dimensions between 1 and 100 nanometers. These magnetoparticles show a response to an external applied magnetic field which causes the movement of magnetic nanoparticles to the desired location and induces heating because of the induced magnetization. Magnetic nanoparticles show superparamagnetism, do not remain magnetized after removal of magnetic field, offer advantage of reduced particles aggregation. At small sizes these particles become a single magnetic domain having a net magnetic moment. In the absence of external magnetic field at higher temperatures there is loss of net magnetization.
Three broad groups of magnetic nanoparticles are :
Oxide-based magnetic nanoparticles
Metallic nanoparticles
Metallic nanoparticles with shell
Oxide-based magnetic nanoparticles commonly consists of two components, a magnetic material, often iron, cobalt, nickel and a chemical functionality say oxygen.
Metallic nanoparticles are purely made of reactive metals which gets easily oxidized and ignites when comes in exposure of air.
In metallic nanoparticles with shell, surface is coated with inert layer of precious metal, surfactants, and polymers or is gently oxidized.
Properties of Oxide-Based Magnetic Nanoparticles
Magnetic nanoparticles have several different properties from those of a bulk specimen. The Curie temperature or Neel temperature and the coercivity field are some of the magnetic properties found to be different from the bulk material.
Small regions in bulk ferromagnetic materials are called magnetic domains. The balance of the energy terms like magnetostatic energy, exchange energy and magnetocrystalline anisotropy leads to the formation of magnetic domains.5,6 The magnetocrystalline anisotropyposition magnetic moment in particular direction and the exchange energyposition all magnetic moments in same direction. Magnetization is eliminated by the magnetostatic energy. These domains have different direction of magnetizations. And addition of these domains resulted into net magnetization. Below a critical size limit ferromagnetic materials acts as single domainparticles.7,8
At specific higher temperature called blocking temperature (TB) these magnetic nanoparticles behave as superparamagnetic having magnetic moment fluctuating around the easy axes of magnetization. As a consequence, each magnetic nanoparticle possesses a large magnetic moment with changing orientation. As the temperature is lowered below blocking temperature, there is lowering of thermal agitation and magnetic moment freeze in random directions.
These nanoparticles have large fractions of atoms at their surface and due to this surface to bulk ratio of atoms is large resulting in significant magnetization.9 Because of surface effects, nanoparticles and their oxides show ferromagnetism. Presence of magnetically dead surface layer decreases the magnetization of some oxide nanoparticles.
Synthesis Methods
Due to new physical and chemical properties of these materials at nanoscale their synthesis has received great interest in recent years. Distinct chemical methods can be applied for the synthesis of magnetic nanoparticles depending upon their shape, size, structure, defects, surface structure and magnetic properties .
Some methods presented here include:
Classical synthesis by Coprecipitation
Synthesis by Hydrothermal Method
Polyol Method
Electrochemical Method
Flow Injection technique
Synthesis by Coprecipitation
Coprecipitation can efficiently synthesize magnetic nanoparticles with specific physical properties. It involves mixing of the metal ions with different oxidation states in particular molar ratio at specific temperature. The reaction can be controlled by pH changes. Coprecipitation is commonly used for precipitating Fe3O4nanoparticles.10,11
Ferric oxide can be formed by the reaction:
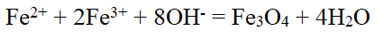
Fe3O4precipitates completely between pH 8.2 to 13.9.
Synthesis by Hydrothermal Method
Due to its simple, economical and efficient mechanism, hydrothermal method has attracted the scientific community for production at large scale.
This method uses high temperature and pressure. The reactions are carried out in closed containers kept above the boiling point of aqueous/polar medium12. Shape and size of the particles can be controlled by ratio of reactants, rate of nucleation, crystal growth and concentrations. A general outline of the hydrothermal method can be given as follows:
 |
Figure 1: Hydrothermal method. |
XRD and FTIR Spectroscopy can be used for continuous measurement of composition and other properties during the process.
Polyol Method
One of the most suitable methods for the uniform nanoparticles synthesis with well-defined shapes and sizes is polyol method. Ethylene glycol, diethylene glycol are most commonly usedpolyols.13,14 High boiling points, high dielectric constants good solvents to dissolve inorganic compounds are some of the interesting properties of these nanoparticles. In this method, a precursor is suspended in liquid polyol and heated to its boiling point. This results in stabilization of the metal precursor in the diol and its reduction to form metal particles.
 |
Figure 2: Synthesis of nanoparticles by polyol method15 |
Electrochemical Method
The synthesis of magnetic nanoparticles by the passage of current through a cathode and an anode placed in an electrolytic solution is called electrochemical method of synthesis. In this method cathode is reduced to metal and anode is oxidized to metal ion, resulting in nanoparticle formation at electrodes-electrolyte interface.
The reaction is regulated by the cell potential as the process is dependent on both the reduction and oxidation reactions. Surfactants are used to avoid segregation. Characterization can be done by XRD, TEM and Infrared Spectroscopy.
 |
Figure 3: Comparative presentation of the synthesis of magnetic nanoparticles by different route16 |
Flow Injection Technique
The adapted form of coprecipitation technique having an advantage of reproducibility and high mixing homogeneity is flow injection technique. It can be employed for magnetic nanoparticles of very small sizes say 2-8nm. Small nanoparticles can be obtained by using emulsion matrices through zone confinement.17,18
Growth and Size Control
Size of a particle and its Growth can be controlled by various physiochemical methods. Some of them being the following:
Charging of the nanoparticles
Coating of nanoparticles surface with suitable species
Zone segregation of nanoparticles
Every synthesis process has unique properties and disadvantages.19Thenet charge present on these particles and electric double layer surrounding them leads to their stability. In polar solvents, these particles can adsorb the ionic species leading to electrostatic forces. Due to the electrostatic forces, particles having similar charges repel each other. Stable scattering occur when repulsive forces overcome attractive forces.20,21
Suitable capping agents can stabilize these particles. The abnormal growth of particles can be stabilized by their division into different zones or by segregation. Scattering of particles in a solid matrix can help in stabilization of the particles. In various applications like catalysis and optical these strategies have been used extensively.22,23,24
Characterization Techniques
In the field of nanoscience, for scientific understanding of engineered nanomaterials their characterization is carried out. Requisite characterization of magnetic nanoparticles is done in order to know the important physical and magnetic properties of the nanoparticles for understanding their performance.25
Characterization of size of nanoparticles is done by following techniques:
Transmission electron microscopy [TEM]
X-ray diffraction [XRD]
Dynamic light scattering [DLS]
Fourier Transform Infrared Spectroscopy
X-Ray Photoelectron Spectroscopy
Selected Area Electron Diffraction [SAED]
Scanning Electron Microscopy [SEM]
Standard measurement techniques used with magnetometers or quantum interface devices provides better measurements. Among the various techniques, DLS tells about how a particle diffuses within a fluid and the diffusion coefficient is the same as that of particle being measured. FT-IR and Raman spectroscopies helps in vibrational characterization of nanoparticles26.
Iron-Oxide Nanoparticles
Magnetite(Fe3O4), maghemite (ɣ-Fe2O3) and hematite (α-Fe2O3) are the common forms of oxide found in nature whose properties have many uses including magnetic inks, magnetic recording media, ferrofluids, and therapeutic agents for cancer treatment. Appropriate engineering of these nanoparticles with various organic and inorganic species produce multifunctional nanoparticles that perform several functions together27. These transition metal oxides are found in nature as aggregates, nanocoating on soil grains and nanoparticles of minerals28.
One of the oldest known forms of the iron oxides is hematite and is extensively found in soils and rocks. Hematite is formed from other iron oxides as it is stable at very high temperature. Magnetite have strongest magnetism among all transition metal oxide. Maghemite is metastable and forms solid solutions with magnetite.29
The crystal of oxides have iron cations lying in octahedral or tetrahedral interstitial sites and oxygen anions in close-packed planes. In hematite, Fe(III) ions occupy octahedral sites in hcp arrangements of oxygen ions. Magnetite and maghemite, have ccp arrangement of oxygen anions. Inmagnetite Fe(III) ionsoccupy both octahedral and tetrahedral sites, and Fe(II) ions lie in octahedral sites making it an inverse spinel structure.
Magnetic behavior of Iron Oxides
Since we can see that iron has four unpaired electrons in its 3d orbitals, it is resulting in its strong magnetic moment. We can say that different magnetic states can arise if an attempt is made to make crystal from iron atoms. In the paramagnetic state, the crystal has a zero net magnetic moment due to random alignment of the individual atomic magnetic moments. If it is compared with ferromagnetic crystal, even without external magnetic field individual magnetic moments are evenly positioned.
While in a ferrimagnetic crystal atoms with different strengths are aligned in anti-parallel manner leading to a net magnetic moment. The crystal will be called anti-ferromagnetic if the anti-parallel magnetic moments are of same strength, and these substances do not have any net magnetic moment.30
Nanoparticles of about 25nm shows superparamagnetic behavior at room temperature. Magnetic particles which are less than5nm are superparamagnetic at room temperature, but their methods of synthesis govern their magnetic behavior strongly. For example: Ferromagnetic Maghemite becomes unstable at high temperature, and loses its magnetic susceptibility with time. Curie temperature of maghemite lies between 800-950K. Particles of maghemite are less than 10 nm in size and are superparamagnetic at room temperature.31 The size dependance and surface treatment leads to the nanostructuring of magnetic materials which can tune their magnetic properties.32
Crystal Structure of Iron Oxides
13 compounds among the 16 different forms of oxides contain iron in oxidation state +3, while the remaining three compounds, namely, FeO, Fe(OH)2, and Fe3O4, contain iron in +2 oxidation state. Crystalline structure is possessed by all oxides, hydroxides, and oxyhydroxides of iron. The iron oxide structures are described below:
Magnetite (Fe3O4)
Magnetite occurs naturally as magnetic ore, known as loadstone. It is an inverse spinel having Fe ions in both +2 and +3 oxidation states making it different from other iron oxides. Magnetite possesses face-centered cubic structure. 30 oxygen ions are arranged in cubic-close packed arrangement in it. There are eight crystal designs present per unit cell.33Its chemical formula can be described as A[AB]O4.34,35
Substitution of Fe²+ions by other metal ions produces spinelsMnFe2O4, NiFe2O4, CoFe2O4, etc.
Maghemite (ɣ-Fe2O3)
Nanoparticles of Maghemite are majorly relevant in nanomedicine because of their excellent magnetic properties. Biocompatibility can be incorporated and low harmfulness can be produced within them. Maghemite is formed by weathering of magnetite. This iron oxide can be described both as a cubic system with disarranged iron vacancy or as tetragonal system. All the iron atoms are in oxidation state (+3). The charge neutrality of the unit cell is maintained by the vacancies of cations. The lattice parameter, a=0.834nm is present in cubic form of maghemite.
In nutshell, maghemite consists of cationic vacancies, which may be ordered or disordered.36
Hematite (α- Fe2O3)
Hematite has splendid implementations in the sphere of splitting of water, storage of magnetic substances, treatment of environment due to its resistance to corrosion, low expense for processing and non-toxicity.37It also serves as a precursor for synthesis of magnetite.
Hematite has hexagonal lattice with unit cells having lattice parameters a=0.5034nm. In a unit cell the number of crystals are six. Hematite possesses structure similar to α- Al2O3(Corundum). The anions are distinguished by systematic consecutive changes in the two layers. Two-third of the octahedral sites are occupied by cations.
Ferrihydrite
Ferrihydrite (Fh) is a substantial ferri-oxyhydroxides mineral present in the earth’s crust. Ferrihydrite also occur in ferritin protein in living beings and its function to store excess iron. Ferrihydrite is a deficiently crystalline iron oxyhydroxides. Its composition is estimated as 5Fe2O3.9H2O.38
Cobalt Ferrite Nanoparticles
Cobalt ferrites are included in the category of “hard ferrites” and possess ample number of applications. These can be used in the field of medicines, permanent magnets and microwave devices.39They have similar crystallization pattern to an inverse spinel like magnetite. The cobalt ions have been replaced by divalent ions in it. The lattice parameter of the unit cell is not altered.
Nickel Oxide Nanoparticles
Nickel oxide (NiO) is a major transition metal oxide. Its magnetic properties have attracted various researchers.40,41 Rhombohedral structure is possessed by bulk crystals of NiO. It showcases antiferromagnetic behavior below 523 K (TN). On the other hand, it has a cubic structure and shows paramagnetic behavior above that temperature.
Over the last few years, nanoparticular nickel oxide has attracted the interest of many researchers due to its magnificent electrochemical properties. Several attempts have been made to synthesize nanosized NiO in controlled sizes and shapes owing to its applications.
Most of the study reports of nanoparticulated NiO have reported the use of the sol-gel method or the co-precipitation method. It is not easy to have uniformity and mono dispersity in such methods. One of the important route for synthesis of nanoparticles is the micro emulsion method.42 Homogeneous and mono-disperse nanoparticles are provided by this route. It is possible to manage the size as well as the morphology of the nanoparticles. Characterization of the NiO nanoparticles is performed by XRD method, TEM, electron diffraction, dynamic light scattering studies, X-Ray photoelectron spectroscopy, FTIR spectroscopy and electrochemical studies.43
Synthesis
Preparation of nickel oxide can be performed in two steps using a reverse micellerpathway utilizing cationic surfactant. First step involved the formation of nickel oxalate dehydrate in form of nanoparticles from emulsions. These nickel oxalate nanoparticles were then subjected to thermal decomposition to yield nanoparticles of nickel oxide.
Characterization
The powder X-ray pattern was found to have a=4.1743 Angstrom. The grain size that was inferred from X-ray was found to be 30nm.
A broad absorption maximum lying between 400-415 cm-1 in FTIR suggests nanocrystalline NiO.
 |
Figure 4: (A). FTIR spectrum of nanostructured NiO in the frequency range 50-500 cm-144 |
 |
Figure 4: (B). FTIR spectrum of nanostructured NiO in the frequency range 50-100 cm-144 |
It can be concluded that almost spherical nanoparticles of nickel oxide which were found to crystallize in the cubic structure were manufactured using the reverse micellar route.44,45The methodology led to the formation of nearly consistent nanoparticles with a tapered size distribution.
Manganese Oxide Nanoparticles
Manganese oxide nanoparticles are important precursors for a number of things. They have many applications in the field of catalysis, electrodes, sensors, molecular adsorption and high density magnetic storage media. An important oxide of manganese is hausmannite (Mn3O4).It has been widely used for reduction of nitrobenzene, oxidation of methane.46Contrasting agents of Mn-oxide based nanoparticles produces high quality MRI due to its bright images and biocompatibility.47
When particle size become comparable to the length of the magnetic interaction its magnetic properties change. This means that the bulk form can have different properties that the nanoparticles. Bulk Mn3O4undergoes the ferrimagnetic transition at a curie temperature of TC = 42K.48
MnO and Mn3O4 nanoparticles were synthesized using various methods.49The non-aqueous synthesis of nanoparticles with varying temperature ramp and aging time provide better control over size and shape of particles49, their crystallinity and surface properties.
Characterization of manganese oxide
The crystallinity of the manganese oxide synthesized by non-aqueous synthesis was investigated by the technique of X- Ray Diffraction.
The magnetic properties of MnO and Mn3O4 nanopowders can be done with the help of temperature dependent EPR study, HRTEM studies.
Thus, it can be concluded that the synthesis of nanoparticles of manganese oxide through different routes and precursors yielded a different phase composition of the oxides being synthesized which can be characterized by various techniques. The nanoparticles which were obtained by this method of synthesis were found to be singular crystalline.
Cobalt Oxide Nanoparticles
Cobalt oxides are very crucial practical materials which have electrochemical, magnetic and catalytic applications. They are used as supercapacitors, as temperature and gas sensors, in enamels, grinding wheels, solar energy absorbers, in dihydrogen gas synthesis, catalysis for decomposition of NO, oxidation of trace ethylene, etc. Innumerable procedures have been devised to synthesize CoO nanostructures.50 Cobalt oxide is very tedious to be obtained in a single phase in pure form. Co3O4 could turn out to be a nice example of pseudocapacitors.51Co3O4 based pseudo capacitor devices are used as substitute storage devices, pulsed power applications, memory backup, equipments of military, flexible biomedical devices.52
Spinel Co3O4 is environment friendly.53 The efficiency to transport electrons and ions in Co3O4 based pseudo capacitors depended on properties such as surface area, morphology etc.54
Synthesis
Solvothermal strategy was used to form stable Co3O4 nanocrystals. The method involved quickest formation method of Co3O4. Two syntheses were carried out. First involving use of Cobalt acetate in ethanol and aqueous sodium hydroxide and reaction time was kept 20h. While the other synthesis also involved the same precursors but the reaction time was increased to 36h.
Characterization
UV-Visible spectrophotometry can be used to procure the optical properties of Co3O4. Cyclic Voltametry (CV) will be helpful in evaluating the performance, which is electrochemical in nature, of the synthesized materials.55
Conclusion
The main idea behind the review was to showcase a few characteristics of magnetic nanoparticles which are oxide-based. These nanoparticles were found important from the basic scientific point of view. Their comprehension is found crucial in order to successfully implement magnetic nanoparticles in biomedical applications such as MRI and magnetic hyperthermia.
In this report we discussed about the properties of magnetic nanoparticles which are important for the synthesizing nanoparticles of particular use. Various oxide-based nanoparticles can be synthesized depending on the properties which are needed for their applications. Varioustechniques can be used to manage the size and growth of the nanoparticles. Among the methods reported, hydrothermal method offers the most promising method for the same.
Different characterization techniques have been listed up which provides substantial knowledge of the crystal structure, shape, size and crystallinity of the magnetic nanoparticles.
It was found that properties of magnetic nanoparticles are considerably different from the bulk materials. Several nanoparticles including iron oxide, manganese oxide, nickel oxide and cobalt oxide nanoparticles have been discussed including their magnetic behavior, most successful method of synthesis, their common characterization techniques and some of their most important applications in biotechnology, electronics, catalysis and storage media.
Acknowledgements
I acknowledge all the co-authors and Maharshi Dayanand university for their constant support.
Conflict of interest
There is no conflict of interest.
References
- Sun, S. ;Murray, C. B.; Weller, D. ; Folks L.; Mose,A.Science.1989, 287, 2000.
CrossRef - Beecroft L. L.; Ober, C. K.Chem. Mater. 1997, 9, 1302.
CrossRef - Pankhurst, Q. A.; Connolly, J.; Jones, S. K.;Dogson, J.J. Phys. D: Appl. Phys,2003, R167, 13.
CrossRef - Leslie-Pelecky D. L.; Rieke, R. D.Chem. Mater.1996, 8,1770-1783.
CrossRef - Wang, W. ;Efrima S.; Regev, O.J. Phys. Chem. B. 1993,103, 5613-5621.
CrossRef - Wilson, K.; Harris, L.; Goff,J.; Riffle, J.; Dailey,J. Eur. Cell. Mater.2002, 3, 206-209.
- Chen, D.; Tang, X.; Wu, J.; Zhang, W.; Liu Q.; Jiang, Y.J.Magn. Magn. Mater.2011,323,1717-1721.
CrossRef - Azcona, P.;Zysler R.;Lassallee,V. A Physiochem. Eng. Asp.2016, 504, 320-330.
CrossRef - Lu, A. H.; Salabas, E. E. L.; Schuth, F.Angew. Chem. Int. Ed.,2007, 46, 1222-1244.
CrossRef - Zysler, R. D.; Fiorani, D.;Esta, A. M.J. Magn. Magn. Mater.2001, 224, 5-11.
CrossRef - Hermanek,M.;Zboril,N.; Medrik, J.;Pechousek,C. G. J. Am. Chem. Soc.2007,129, 10929-10936.
CrossRef - Agrawal, N.; Munjal, S.; Ansari, M.; and Khare, N.; Ceram. Int., vol. 43, 2017, doi: 10.1016/j.ceramint.2017.07.176.
CrossRef - Teja A. S.; Koh, P. Y.J. Mater. Sci. Appl.2009, 55, 22-45 .
CrossRef - Anderson, W., Kozak, D.; Coleman, V.;Jamting, A.; Trau, M.J. Coll. Inter. Sci., UK, 2013, 405, 322-330.
CrossRef - Dong, H; Chen, Y.C.; and Feldmann, C; Green Chem.17, 2015,41078-4132.doi: 10.1039/C5GC00943J
CrossRef - Ali, A; Hira Zafar, M.Z.; ulHaq, I.; Phull, A, R.; Ali, J.S.; and Hussain, A.; Nanotechnol. Sci. Appl. 9:49, 2016, doi: 10.2147/NSA.S99986
CrossRef - Massart,R.IEEE Trans.Magn.1981, 17, 1247-1248.
CrossRef - Sugimoto, T. CRC Press, Boca Raton, FL, 2000.
- Laurent, S.; Forge,D.; Port, M.; Roch, A.; Robic, C.; Vander, E. L.Muller, R. N.Chem. Rev.2008, 108, 2064-2110.
CrossRef - Pankhurst, Q.; Thanh, N.; Jones,S.; Dobson, J.J. Phys. Appl. Phys.2009,42, 24001.
CrossRef - Cornell, R. M.; Schwertmann, U. Second ed. Wiley-VCH, Weinheim. 2003.
- Neuberger, T.; Schopf, B.; Hofmann,H.; Hofmann, M.; Rechenberg, V.J.Magn. Magn. Mater.2005, 293, 483-496.
CrossRef - Spada, F. E.; Parker, T. F.; Nakakura, C. K.; Berowitz,A. E. J.Magn. Magn. Mater.1993, 120, 129-135.
CrossRef - Cornell R. M.;Schwertmann, U.John Wiley & Sons, Hoboken, NJ, 2003.
- Gossuin,Y.; Gillis,P.;Hocq,A.;Vuong,Q. L.; Roch,A. WiletInterdiscip Rev NanomedNanobiotechnol.2009, 1, 299-310.
CrossRef - Khan, I.; Saeed, K.; Khan, I.; Arabian Journal of Chemistry, 2019, 12, 908-931.
CrossRef - Tran, V., H.; Ngo, M., N.; Medhi, R.; Srinoi, P.; Liu, T.; Rittikulsittichai, S.; Lee, R., T.; Journal of Materials, 2022, 15(2), 503.
CrossRef - Claudio, C; Iorio, E; Liu, Q; Jiang, Z; Barron, V; Journal of Nanoscience and Nanotechnology. 2017;17(7):4449-4460.
CrossRef - Rohrer,G. S. Cambridge University Press, Delhi, 2001.
- Shimomura,Y.;Tsubokawa,I.Kojima, M.J. Phys. Soc. Japan.1954, 521.
CrossRef - Luisi, P. .L.; Straub, B. E. Plenum Press, New York and London.1984,654.
- Kuiry, S. C.; Seal, S.; Nalwa, H. S. American Scientific Publishers, 2004, 369.
- Yang,L. X.; Zhu, Y. J.; Tong,H.; Wang, W. W.; Cheng, G. F.J. Solid State Chem.2006,179, 1225-1229.
CrossRef - Seo, W. S.; Jo,H. H.; Lee, K.; Kim, B.; Oh S. J.; Park, J. T.Angew.Chem. Int. ed., 43, 2004, 1115-1117.
CrossRef - Hendriksen, P. V.;Linderoth, S.; Lindgard, P. A. Phys. Rev. B, 1993,10, 7259.
CrossRef - Wu, C.; Yin,P.; Zhu, X.; Ouyang, C.; Xie, Y. J. Phys. Chem. B, 2006,110, 17806-17812.
CrossRef - Ngo, A.;Pileni, M. J. Phys. Chem. B, 2001,105, 53-58.
CrossRef - Moskowitz, B.; Raj, K.;Casciari, R.J. Magn.Magn. Mater.1995, 149, 174-180.
CrossRef - Tartaj, P.; Gonzalez, C.; Serna, C. J.Adv. Mater.2001, 13, 1620.
CrossRef - Smart, J. S.; Greenwald, S.Phys. Rev.1951, 82, 113.
CrossRef - Kodama,R. H.; Makhlouf, S. A.; Berkowitz, A. E.Phys. Rev. Lett.1997, 79,1393.
CrossRef - Cinnsealach, R.; Boschloo,G.; Rao, S. N.; Fitzmaurice,D. Sol. Energy Mater. Sol. Cells.1999,57,107.
CrossRef - Kitao, M.; Izawa,U. K.; Komatsu, S. T.;Kuwano,S. Y. J. Appl. Phys. Jpn.1994, 33, 6657.
- Niederberger, M.; Garnweitner, G.Chem. Eur. J.2006, 12, 7282-7302.
CrossRef - Djerdj, I.; Acron, D.; Jaglicic, Z.; Niederberger,M. J. Phys. Chem. C.2007,111, 3614-3623.
CrossRef - Cai, X.; Zhu, Q.; Zeng, Y.; Zeng, Q.; Chen, X.; Zhan, Y.; International Journal of medicine,2019, 14,8321.
CrossRef - Ngo, A. ;Pileni, M.J .Phys. Chem. B.2001,105, 53-58.
CrossRef - Torre, M., C.; Grossman, H., J.; Bobko, A., A.; Bennewitz, M., F.; J. PLoS ONE, 2020, 15(9):e0239034.
CrossRef - Ujjain, S. K.; Deori, K.; Sharma, R. K.; Deka, S.ACS Appl. Mater. Interfaces.2013,21, 10665-10672.
- Chen, S.; Zhu, J.; Wang, X. J.Phys. Chem. C.2010,114, 11829-11834.
CrossRef - Li, H. B.; Yu, M. H.; Wang, F. X.; Liu, P.; Liang, Y.; Xiao, J.; Wang, C. X.; Tong, Y. X.; Yang, G. W.Nature Commun.2013.
- Wu, J. B.; Lin, Y.; Xia, X. H.; Xu, J. Y.; Shi, Q. Y.Electrochim. Acta.2011,56, 7163-7170.
CrossRef - Wang, Y.; Zhong, Z.; Chen, Y.; Ng, C. T.; Lin, J. J. Nano Res.2011, 4, 695-704.
CrossRef - Deori, K.; Ujjain, S. K.; Sharma, R. K.; Deka, S.ACS Appl. Mater. Interfaces. 2013,21,10665-10672.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









