Sorption Capacity of Toxic Heavy Metal Cr (VI) Ion on bentonite clay from Aqueous Solution by Kinetic and Thermodynamic Studies
Priyanka Rathore* and Rashmi Verma
Department of Chemistry, Dr. C.V. Raman University Kota Bilaspur (C.G.) India.
Corresponding Author E-mail: mdazraali@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370512
Article Received on : 03-Jun-2021
Article Accepted on :
Article Published : 09 Sep 2021
Reviewed by: Dr. Yashas S R

Second Review by: Dr. M.A Shah
Final Approval by: Dr. Dyah Sari
The present study removal of toxic heavy metal Cr (VI) ion on bentonite clay from aqueous solution under different experimental conditions and also study sorption capacity are effect different pH, concentration, temperature and contact time of shaking.My study focused on use of bentonite clay as a best adsorbent for the removal of toxic heavy metal Cr (VI) ions from aqueous solution. Initial metal ions concentration increases 50 to 250mgL-1 the % removal Cr (VI) ion decreases from 66.64 % to 53.94 % but amount of sorption increases from 0.833 to 3.371mgg-1, The particle size of bentonite clay increase from 45 to 150μ by amount of adsorption of Cr (VI) decreasing from1.589 mgg-1 to 1.153mgg-1 , pH increasing from 2.0 to 10.0 the amount of sorption decrease from 2.018 to 1.161 mgg-1, temperature increasing from 303K to 313K adsorption is also increases from 1.589 to 1.822mgg-1. Kinetic study for value of rate constant k1 and k2 is favour concentration increases the amounts of the metals sorbent qcal also increases. Q and kf sorption capacity related to Langmuir and Freundlich adsorption isotherm constant. Value of both is favour sorption is temperature dependent. Thermodynamic study the value of ΔG0 increase -1.402 to -2.655it is best evidence temperature increases from 303K to 323K the adsorption of Cr (VI) ion increases because value of ΔG0 is negative favour the adsorption. My investigation best evidence the value of kinetic, equilibrium and thermodynamic parameter also favours the Sorption of Cr (VI) ion on bentonite clay from aqueous solution.
KEYWORDS:Effect of pH; Equilibrium; Kinetic; Removal of Toxic Heavy Metal ion; Sorption Capacity of bentonite; Thermodynamic Study;
Download this article as:| Copy the following to cite this article: Rathore P, Verma R. Sorption Capacity of Toxic Heavy Metal Cr (VI) Ion on bentonite clay from Aqueous Solution by Kinetic and Thermodynamic Studies.Orient J Chem 2021;37(5). |
| Copy the following to cite this URL: Rathore P, Verma R. Sorption Capacity of Toxic Heavy Metal Cr (VI) Ion on bentonite clay from Aqueous Solution by Kinetic and Thermodynamic Studies.Orient J Chem 2021;37(5). Available from: https://bit.ly/3nfOh2L |
Introduction
Chromium is a fundamental supplement for plants and creature digestion. Chromium species is basically Cr3+ and Cr6+ distinctive two oxidation states in aqueous solution. Cr (III) is considered as a fundamental minor component for the support of successful glucose, lipid and protein digestion in warm blooded creatures1. On the other hand, Cr (VI) which is present as either dichromate (Cr2O72-) in acidic environment or chromate (CrO42-) in alkaline environments can be toxic for biological systems and carcinogenic for both humans and animals2. Therefore the Poisonousness and natural movement of the Chromium relies upon the aggregate sum as well as on its compound form3. Chromium in its hexavalent structure being harmful is brought into the water bodies from various mechanical cycles like tanning, electroplating, metal preparing, paint producing, steel creation and agrarian runoff4. As per USEPA the greatest reasonable cutoff points in versatile and squander water for chromium is 0.05 and 1.0 ppm respectively5. Adsorption techniques are viably and financially among different techniques to eliminate toxic heavy metals from aqueous solution 6. The project of the study to effect of sorption depend of metal ion concentration on bentonite clay at other parameter like pH (2), temperature (303K) and particle size (45μ) are constant. Initial metal ions concentration increases 50 to 250mgL-1 the % removal Cr (VI) ion decreases from 66.64 % to 53.94 % but amount of sorption increases from 0.833 to 3.371mgg-1 The particle size of bentonite clay increase from 45 to 150μ by amount of adsorption of Cr (VI) decreasing from1.589 mgg-1 to 1.153mgg-1, pH increasing from 2.0 to 10.0 the amount of sorption decrease from 2.018 to 1.161 mgg-1, temperature increasing from 303K to 313K adsorption is also increases from 1.589 to 1.822mgg-1. Parameter calculated kinetic equation and equilibrium adsorption isotherm. Kinetic study for value of rate constant k1 and k2 is favour concentration increases the amounts of the metals sorbent qcal also increases. Q and kf sorption capacity related to Langmuir and Freundlich adsorption isotherm constant. Value of both is favour sorption is temperature dependent. The thermodynamic investigate adsorption capacity of bentonite clay to removal of Cr (VI) metal Ion. In order of determine the thermodynamic feasibility2 and the thermal effect of the adsorption. Table -3 the value of (ΔG0), (ΔS0) and (ΔH0) will be calculated for study at different temperature from 303K to 313K. The values ΔG0 are negative the favour sorption process is spontaneous, ΔHo Value positive indicate adsorption nature is endothermic and ΔS0 value positive indicates affinity of the bentonite clay for the Cr (VI) ions.
In the present study adsorption capacity of bentonite clay for the removal of Cr (VI) ions has been analysed at different pH, concentration, temperature and contact time of shaking was examined. To the best of the knowledge of authors, bentonite has not been used before for the removal of Cr (VI) ion using present parameters.
Materials and methods
All chemical used our investigation of analytical reagent (A.R.) or laboratory reagent (L.R.) grade. Potassium Dichromate (98.20%), Sodium hydroxide and sulphuric acid (99%w/w) supplied by molly chem. Limited Mumbai by help of Javed trading raigarh (C.G.) Product code 17120, distilled water used in preparation of bentonite Clay supplied by Titan biotech ltd. Bhiwandi.Utilize 25mL of aqueous solution of Cr (VI) with 1.0g of sorbent in various glass bottles at various temperature (303K, 313K, and 323K), pH (2.0, 4.0, 6.5, 8.0 and 10.0), metal ion concentration (50, 100, 150, 200 and 250 mg/L) and particle size (45, 75 and 150 μ) in water both shaker machines. 0.05 M NaOH and 0.05 M HCl solution ate use adjusting of pH by pH meter. Every 20 min. was noted progress of sorption. Toward the finish of pre decide fixed time interim. After 160min sorbate and sorbent were isolated by centrifugation machine and the supernatant fluid of solution than analyzed by AAS.
Result Discussion
Effect of Concentration
Fig.-1 Represents percentage removal of Cr (VI) ion versus initial Cr (VI) metal ion concentration. Graph study increase from 50mgL-1 to 250mgL-1 metal ion concentration the % removal Cr (VI) ion decrease from 66.64 % to 53.94 %. Because adsorption possesses a “limited number of active sites and theses active sites become saturated at certain concentration”7
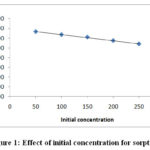 |
Figure 1: Effect of initial concentration for sorption. |
Fig-2 Represents increase metal ion concentration the amount of adsorbent at qe mgg-1 increase as increase from 50 mg/L to 250 mg/L metal ion concentration the amount of adsorbent increase from 0.833 mgg-1 to 3.371 mgg-1. “Because due to the fact that mass transfers resistance of Cr (VI) ion between aqueous and solid phase is possibly overcome by the initial metal ion concentration”8.
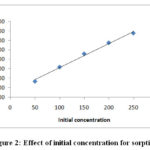 |
Figure 2: Effect of initial concentration for sorption. |
Effect of Contact Time
Fig-3 evidences that amount adsorption increase with increase time in minute. The amount of sorption increases with contact time rapidly in the initial stage and become slowly in the later stages till equilibrium it evaded from resultthe time required to accomplish equilibrium for the sorption is 160min. It can see that the time of equilibrium is independent of initial concentration of metal ion. “Initial the rate of sorption if fast which gradually slows down till equilibrium is reached”2.7.
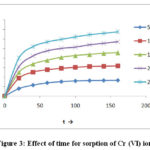 |
Figure 3: Effect of time for sorption of Cr (VI) ion |
Effect of pH
Adsorption is highly depending on pH of the solution. Cr (VI) is highly adsorption in high acidic medium. It is proved by this observation the adsorption of Cr VI metal ion decreases with increases pH of the system. Every single metallic ions of degree of hydrolysis depend on pH of the solution. Fig-4evidences pH increasing from 2.0 to 10.0 the amount of adsorption decrease from 2.018 to 1.161 mgg-1 because decrease pH negative Cr (VI) ion absorption is easily2.
 |
Figure 4: Effect of pH for adsorption. |
Effect of temperature
Fig-5It is proved by this observation the adsorption of Cr (VI) metal ion increases with increases temperature of the system. Mobility of metal ion increases as temperature is increase.Along these lines probability of diffusion of Cr (VI) metal ions in pores of clay increases. Pore size likewise increases with temperature. Diffusion forms is endothermic in nature should increases with rise in temperature. It watched information is accordance with this. Along these lines it very well may be presumed that rise of temperature supports’ the sorption. The increasing temperature from 303K to 313K amount of adsorption is also increases from 1.589 to 1.822mgg-1 at 303K, 313K and 323K are increase temperature with respect indicate the amount of adsorption increase2.10.
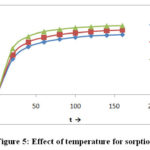 |
Figure 5: Effect of temperature for sorption |
Effect of particle size
Fig-6 the particle size of bentonite clay on adsorption of Cr (VI) ion is affected. It proofed from figures and tables the size of sorbent particle increases from 45μ to 150 μ as the amount of adsorption of Cr (VI) decreases from1.589 mgg-1 to 1.153mgg-1 because size of bentonite particle increases its surface area decreases consequently the number of active sites decreases as a result sorption decreases
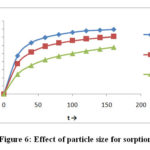 |
Figure 6: Effect of particle size for sorption |
Kinetic studies
To explain the adsorption kinetics, pseudo-first-order kinetic equation will be applied to the experimental data the linearized form of the well known Lagergren equation for adsorption kinetic at different concentration is as below:-
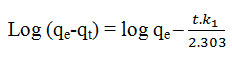
Where qe are the amounts of the metals sorbent at equilibrium and qt are the amounts of the metals sorbent time “t” respectively and k1 is the rate constant of sorption can be determined experimentally by plotting graph log (qe-qt) versus t.
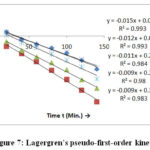 |
Figure 7: Lagergren’s pseudo-first-order kinetic. |
The pseudo-second-order kinetic is given as
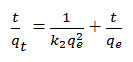
Where k2 is the rate constant of the pseudo second order equation can be calculate experimentally by graph plotting of t/qt versus t5.
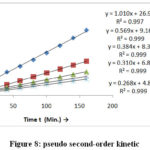 |
Figure 8: pseudo second-order kinetic. |
Table 1: Kinetic parameter for sorption of Cr (VI) ion on bentonite Clay
|
Con. mgL-1 |
Lagergren-first-order |
Pseudo-second-order |
|||||
|
K1 (min-1) |
qcal (mgg-1) |
qexp (mgg-1) |
R2 |
K2 (gmg-1min-1) |
qcal (mgg-1) |
R2 |
|
|
50 |
3.45 X 10-2 |
1.040 |
0.833 |
0.993 |
3.785 X 10-2 |
0.990 |
0.997 |
|
100 |
2.76 X 10-2 |
1.172 |
1.589 |
0.993 |
3.531 X 10-2 |
1.757 |
0.999 |
|
150 |
2.53 X 10-2 |
1.871 |
2.287 |
0.984 |
1.756 X 10-2 |
2.604 |
0.999 |
|
200 |
2.07 X 10-2 |
1.914 |
2.869 |
0.98 |
1.412 X 10-2 |
3.226 |
0.999 |
|
250 |
2.07 X 10-2 |
2.143 |
3.371 |
0.983 |
1.466 X 10-2 |
3.731 |
0.999 |
Fig-7 and Fig-8 and table-1 give the kinetic model result high correlation coefficient obtained for the pseudo second order kinetic all the studies concentration in the comparison pseudo second order kinetic model are better explained by first order kinetic model. Experimental data qe(cal) value better qe(exp.) and K2 constant with increase in metal ion concentration.The explanation behind this might be plausibility of lower rivalry for surface dynamic locales of adsorbent at lower concentration. As the metal particle concentration increase surface active sites increase with decreases the rate.
Equilibrium studies
Langmuir adsorption isotherm modal
Experimental equilibrium data obtained will be discussed using Langmuir adsorption isotherm modal accepts sorption on a “homogeneous surface by monolayer sorption without collaboration between adsorbed molecule the linear type of Langmuir isotherm which is as underneath”13
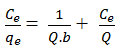
Where Ce (ppm) is equilibrium concentration of Cr (VI) ion, Q and b are constant indicating the sorption capacity and energy respectively. Q and b calculate slope and intercept of the straight line graph plotting between Ce/qe Vs Ce.
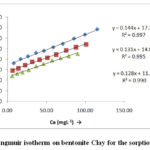 |
Figure 9: Langmuir isotherm on bentonite Clay for the sorption of Cr (VI). |
The Freundlich isotherm model
For sorption on heterogeneous surface this model is used. It’s linearized from is represent as
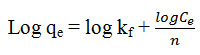
Where kf and n is freundlich constant related to sorption capacity and intensity of the sorbent14 the value of kf and n calculate slope and intercept of the straight line graph plotting between log qe verses log Ce is linear. It indicates the applicable of the above model.
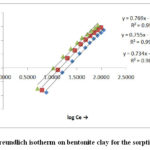 |
Figure 10: Freundlich isotherm on bentonite clay for the sorption of Cr (VI). |
The present fig 9-10 and table-2 the high correlation coefficients R2 values are shown by isotherm Frendlich equation than Langmuir equation. It may be concluded that the experimental data fits in Langmuir isotherm but Frendlich isotherm data fits better for bentonite clay. Besides as 0 < Q it indicate that adsorption process is favourable normally increases in temperature adsorption capacity Q increase which indicate high adsorption it has been observed. Similarly as temperature increases adsorption intensity b also increases. Various data regarding Q and n have been table-2
Table 2: Sorption isotherm constant on bentonite clay for sorption of Cr (VI) ion
|
Temp. (K) |
Langmuir Isotherm Parameter |
Freundlich Isotherm Parameter |
||||
|
Q |
b |
R2 |
n |
kf |
R2 |
|
|
303 |
6.944 |
0.833 X 10-2 |
0.997 |
1.303 |
9.64 X 10-2 |
0.993 |
|
313 |
7.634 |
0.933 X 10-2 |
0.995 |
1.306 |
11.56 X 10-2 |
0.994 |
|
323 |
7.812 |
1.108 X 10-2 |
0.990 |
1.323 |
13.96 X 10-2 |
0.994 |
Thermodynamic study
In order of determine the thermal effect and thermodynamic feasibility of the adsorption2. The Gibb’s free energy (ΔG0), the entropy (ΔS0) and the enthalpy (ΔH0) will be calculated for study at different temperature from 303K to 313K using following equation
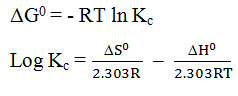
Were Kc is the equilibrium constant. ΔG0 has been calculated from the above equation. Plot graph between logKc verses 1/r gives strait line. The value of ΔH0 and ΔS0 by various graphs slope and intercept of this line. The value of ΔH0 and ΔS0 all value listed table -3. The process is endothermic and exothermic nature is concluded by the sign of ΔH. If process is endothermic the value of ΔH positive but sing of ΔH is negative it show the process is exothermic in nature. The ΔG Gibb’s free energy change is important parameters. If value of ΔG is negative the processer is more effective at higher temperature because increases in temperature the negative value of ΔG decreases, the increases in temperature.
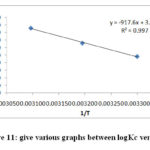 |
Figure 11: give various graphs between logKc verses |
Activation energy
Using modified Arrhenius type equation the value of activation energy and sticking probability have been determined from the experimental data related to surface coverage θ as follows
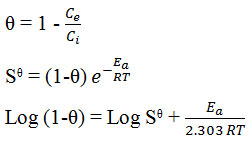
Where the term have their typical significance the sticking probability Sᶿ is a function of the adsorbate/adsorbent system under consideration depending on temperature and should satisfy the condition 0 < Sᶿ << 1. The value of Ea and Sᶿ can be determined from the plot of graph log (1-θ) versus 1/r gives straight line. Slope and intercept of this straight line gives the value of Ea and Sᶿ.
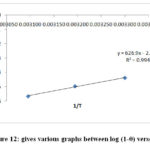 |
Figure 12: gives various graphs between log (1-θ) verses |
Table 3: Thermodynamic study on bentoniteclay for sorption of Cr (VI) ion
|
Temp. K |
ΔG0 (kJmol-1) |
ΔH0 (kJmol-1) |
ΔS0 (Jmol-1) |
Ea (kJmol-1) |
S* (JKmol-1) |
|
303 |
-1.402 |
17.569 |
62.55 |
12.003 |
3.133 X 10-3 |
|
313 |
-1.976 |
||||
|
323 |
-2.655 |
Conclusion
In present examination sorption process has been employed for the removal of Cr (VI) on bentonite. These investigations are effect of pH, temperature, concentration, particle size and contact time on the sorption of heavy metal ion on bentonite. Sorption studies at different initial concentration of sorbate show that the amount adsorbed increases but percentage amount adsorbed decreases with the increase of initial concentration.Adsorption is highly depending on pH of the solution. Cr (VI) is highly adsorption in high acidic medium. It is proved by this observation the adsorption of Cr VI metal ion decreases with increases pH of the system.Normally particle size increases value of qe decrease. Temperature has stamped impact on adsorption the rate as well as percentage adsorption increases with the increase of temperature The calculated data proved that the value of ΔG is negative show that process is spontaneous. The processer is endothermic nature because value of ΔH is positive. The affinity of the adsorbent towards the adsorbate species the value of ΔS is positive. They also supported the positive value of Ea adsorption process is endothermic nature. The adsorption process I mixed process physical and chemical adsorption it is proved by the positive value of activation energy. That the probability to stick on surface of clay is very High because Sᶿ<<1. From the above observation and discussion it may be concluded that bentonite clay are best adsorbent under specific condition.
Acknowledgement
Authors are thankful to Dr. R. P. Dubey (V.C.) and Sri. G. Shukla (Registrar) Dr. C.V. R. U. Kota, Bilaspur (C.G.) for their support. Authors are also thankful to Dr. Mohd Talha, M.G. Govt. P.G. College Kharsia Dist. Raigarh (C.G) and Dr. Dhanesh Singh and Dr. Ajmat Ali, K.G. Arts and Sc. College Raigarh (C.G) for their co-operation and constant encouragement.
References
- Avudainayagam, S.; Megharaj, M. Reviews of Environment contamination and Toxicology. 2003, 178, 53–91.
CrossRef - Ali, A.; Singh, D.; Rathore, P. Our Heritage J.2019, 67, 1244-1252.
- Shanker, A. K.; Cervantes, C. Environment International, 2005, 31, 739–753.
CrossRef - Singh, H. P. Environmental Chem. Letters. 2013, 11, 229-254.
CrossRef - López-Zavala, M. A.; Romero-Santana H. Journal of Water Process Engineering.2020, 38, 422-435.
CrossRef - Ahmad, A.Desalination. 2009,247, 636-646.
CrossRef - Agarwal, M.; Singh, K. J. of Water Reuse and Desalination. 2017, 7, 387–419.
CrossRef - Singh, D. I. J. Chem. Technology.1995, 2, 49-50
CrossRef - Mishra, A.; Dubey, A. I. J. Env. Sc. Tec.2015, 12, 1415-1426.
CrossRef - Chen, H. J.; Weng, W. J. Mater. Chem.2014, 2, 12561-12570.
CrossRef - Kumar, S.; Singh, D. Res. J. Recent Sci. 2014, 3, 18-27.
- Lagergren, S. 1898, 24, 1-39.
- Ali A.; Singh D.; Kumar S.; Verma R.; Rathore P. J. of Emerging technologies and innovative Res.2019, 6, 131-137.
- Das, B.; Monda, N. K. universal J. of Environmental Res. and tech.2011, 1, 515-530.
- Ahmad, A.Desalination. 2009,247, 636-646.
CrossRef - Arivoli, S.; Hema, M.Int. J. Phys. Sci.2007 2, 10-17.
- Devaprasath, P. M.; Solomon, J. S.; Thomas, B.V.J. of Applied Sciences in Environmental Sanitation.2007, 2, 77-83.
- Kumar, S.; Singh, D.; Upadhyay, M.; Mishra, A. K.; Kumar, S.2014 Res. J. Chem. Sci. 2014, 4, 44-53.

This work is licensed under a Creative Commons Attribution 4.0 International License.









