COVID-19: Challenges, Preventive Measures and Remediation
Sameena Mehtab1 , Pragati Joshi1, M. G. H. Zaidi1*
, Pragati Joshi1, M. G. H. Zaidi1* , T. I. Siddiqui2 and Vivek K. Mishra2
, T. I. Siddiqui2 and Vivek K. Mishra2
1Department of Chemistry, G.B. Pant University of Agriculture and ; Technology,Pantnagar, Uttarakhand, India-263145
2Department of Chemistry, D.I.T University, Mussoorie Diversion Road, Makkawala, Dehradun, Uttarakhand, India-248001
Corresponding Author E-mail: mghzaidi@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370204
Article Received on : 10-Sep-2021
Article Accepted on :
Article Published : 08 Apr 2021
This review highlights origin, symptoms, diagnosis, causative agents, mode of transmission and persistence of coronavirus disease (COVID-19) through human activities. In the current scenario researchers, doctors and scientists are striving to combat the transmission of COVID-19 among society. Origin and growth of pandemic has raised the serious concern to address the causative agents, mode of transmission, persistence, preventive measures, diagnosis and possibilities of treatment. The broad-spectrum antiviral antiphrastic, complementary and alternative medicines are currently proposed for possible eradication of the pandemic. The current world is relying on, prevention and control of infection through sanitization and quarantine and onwards waiting for appropriate virucidal agents, sanitizers and strategic vaccination and immunization to combat the pandemic of SARS-CoV-2. Eradication of COVID-19 under stringent guidelines of WHO regarding social practices, intensive care, administration of complementary medicines and innovation of vaccines are under active considerations across the world to combat the pandemic.
KEYWORDS:COVID-19; Causative Agents; Preventive Measures; Persistence; SARS-CoV-2; Transmittance
Download this article as:| Copy the following to cite this article: Mehtab S, Joshi P, Zaidi M. G. H, Siddiqui T. I, Mishra V. K. COVID-19: Challenges, Preventive Measures and Remediation. Orient J Chem 2021;37(2). |
| Copy the following to cite this URL: Mehtab S, Joshi P, Zaidi M. G. H, Siddiqui T. I, Mishra V. K. COVID-19: Challenges, Preventive Measures and Remediation. Orient J Chem 2021;37(2). Available from: https://bit.ly/2PE9ftM |
Introduction
Viruses are minuscule parasites and are associating links of living and non-livings. They don’t have any fundamental activities outside of a living cell. Nonetheless, when it interacts with a vulnerable cell, it infects the cell and controls the cell machinery by blending its hereditary material with the host nucleic acid and begins to reproduce. The hereditary information of viruses could be double or single stranded DNA or RNA. Virion is single virus particle composed of nucleic acid encapsulated into an external shell of protein called capsid. A simplest virus can encode only four while the most complex one can up to 200 proteins. Nature has discovered two essential methods of organizing the various capsid protein subunits and the viral genome into a nucleocapsid1. The tobacco mosaic virus (TMV) is an exemplary case of the helical nucleocapsid. In TMV, the protein subunits appear as disk-like structures with helical shell of a long rod like virus.
Epithelial diseases such as herpes simplex, chickenpox, shingles, and Kaposi’s sarcoma are caused by Herpes viruses occupying double-stranded DNA 2. Whereas Gemini viruses and papilloma viruses have single-stranded DNA that induce cancer and infects many economically important crops 3. The Variola viruses have a super secondary jelly roll fold DNA structure that evokes the most infectious diseases of twentieth-century smallpox, which triggered the discovery of vaccines 4. The most deadliest diseases that emerged before COVID-19 are antonine plague (AD 165), plague of Justinian (541-542), black death (1346-1353), third cholera (1852), flu (1889-1890), sixth cholera (1910-1911), Spanish flu (1918), Asian flu (1957), Hong Kong flu (1968) and HIV-AIDS (2005-2012).
COVID-19 is caused by SARS-CoV-2, that is a non segmented single-stranded RNA with a length ranging 15 to 300 nm which is the longest genome in RNA virus family 5. It is an enveloped structure with non segmented, pleomorphic or spherical particles of size ranging 150 to 160 nm. Their morphological structure comprise crown like projections involving spikes of protein 6. Presence of virions that is the infectious form of virus is revealed through electron microscopy 7. Early, the virus was identified as 2019-novel corona virus (2019-nCoV) that is presently known as the SARS-CoV-2. CoV is the seventh member belonging to the family of corona viridae, a βCoV of group 2B with over 70% similarities in genetic sequence to SARS-CoV-2 8. After comparing SARS-CoV-2 in other vertebrates, the study found that the bat CoV had the most similar infection patterns to the novel SARS-CoV-2 9, 10. The current review presents salient facts regarding morphology, virology, transmission routes, clinical signs, symptoms, current scenario and ongoing practices to control the COVID-19 11, 16.
Mode of Transmission
SARS-CoV-2 were first observed in the early 1960s and found to be responsible for upper respiratory tract infection. SARS-CoV-2 can be grouped into four categories alpha, beta, gamma and delta 11. Bats 12, domestic fowl 13, cattle, cats, dogs, ferrets14, hedgehogs, mink, pigs, rabbits, rats, whale 15 are the key reservoirs responsible for transmission of variety of CoVs (Table 1). To date several SARS-CoV-2 have been identified which includes human SARS-CoV-2 like HCoV-NL63, HCoV-OC43 and MERS-CoV, SARS-CoV and the emerging SARS-CoV2 16. SARS-CoV-2 or 2019-nCoV is considered as cause of COVID-19 generation. Studies during 2002–2003, reveals that bats were the natural reservoir of a range of SARS-CoV-2 and that rhinolophid bats harbour viruses closely related to SARS-CoVs causing respiratory illness in mammals. The infection probably originated in bats as they harbour a wide range of CoV 17, a mutation in the viral spike glycoprotein could have favored the cross‐species transmission from bats to human 18.
Table 1: Key reservoirs and mode of transmission of variety of CoVs
|
Animals |
Variety of CoV |
Ref. |
|
Bats |
Rhinopohus bat CoV (HKU2), Tylonycteris bat CoV (HKU4), Pipistrellus bat CoV (HKU5), ‘Miniopterus bat CoV (1 and HKU8), Decacovirus bat CoV (HKU 10), Rousettus bat CoV (GCCDC1 and HKU), Colacovirus bat CoV (CDPHE 15), Hp-beta CoV (Zhejanj 2013), Rhinolophus ferrumeqinum bat CoV (HuB-2013) and Scotophinus bat CoV 512. |
16 |
|
Domestic Fowl |
Infectious bronchitis virus (IBV)-avian infectious bronchitis, Bulbul coronavirus HKU11, Common moorhen coronavirus HKU21,Munia coronavirusHKU13, Night heron coronavirus HKU19, Turkey coronavirus (TCV)-enteritis, White -eye coronavirus HKU-16, Wigeon coronavirus HKU20 |
17 |
|
Cattle |
Bovine coronavirus(BCV) -severe enteritis in calves |
18 |
|
Dogs |
Canine coronaviruses (CCoV)-enteritis and respiratory diseases |
18 |
|
Ferrets |
Ferret enteric coronavirus-epizootic catarrhal enteritis; Ferret systemic coronavirus-a syndrome similar to feline infectious peritonitis |
18 |
|
Hedgehogs |
Hedgehog coronavirus 1 |
19 |
|
Mink |
Mink coronavirus 1 |
19 |
|
Pigs |
Porcine coronavirus HKU15-gastroenteritis; Porcine epidemic diarrhea virus (PED or PEDV) |
19 |
|
Rats |
Lucheng Rn rat coronavirus |
19 |
|
Whale |
Beluga whale coronavirus SW1 |
19 |
Since Dec 2019, the outbreak of COVID-19 infection was emerged and WHO has declared the public health emergency 30 January 2020. Transmission of SARS-CoV-2 was globally accelerated around February 2020 that has trolled millions of death cases across the world by March 202019,20. Most crucial step for controlling the pandemic is to identify its mode of transmission and infestation into human being. Civilizations has been learning about the mode of transmission, severity of illness and propagation of SARS-CoV-2.The SARS-CoV-2 spread via transmission of droplets produced through human sneezing. Spread of dry cough through spitting and contact among infected patient are the additional cause of propagation of COVID-19. There are three conditions for its transmission that is source of infection, transmission route and sensitivity 21. Virus enters the human body through the mouth, nose and eyes that makes advisable to avoid physical contacts. For the patients with flu and common cold symptoms, WHO has advised to follow the strict preventive measures against the spread of COVID-19 through social isolation. Additional need of joint ventures between public activities and government regulations are of paramount importance to prevent the society from COVID-19 (Fig. 1).
 |
Figure 1: Protective measures against COVID-19 |
Symptoms and Diagnosis
Incubation period of SARS-CoV-2 is an average 5 to 6 days that may extend to 14 days with mild to severe symptoms. Occasionally, the diagnoses of symptoms are difficult leaving the people to be asymptomatic. The SARS-CoV-2 resembles in symptoms with influenza virus that vary by age and immune status. Person with pre-existing medical conditions and old age are found to be more susceptible for COVID-19 22,23. The infection has major health implications interfacing concepts of immunology and respiratory medicine. Major health imperfections arise in infected mammals due to infestation of COVID-19 are respiratory, gastrointestinal, hepatic, and central nervous system. The initial symptoms include respiratory tract illness, like common cold, headache, sore throat, fever, fatigue, loss of taste and smell. In severe cases, patients may suffer from pale appearance of skin, conjunctivitis, diarrhoea, pneumonia, organ failure and sometimes approach to death 24. Reverse rapid transcription polymerase chain reactions (RT-PCR) is employed for qualitative identification of virus based on their genetic fingerprinting. Antigen test is employed to identify the acute or early infections of SARS-CoV-2, while antibody testing evaluates the past history of infection in patients through blood. As per existing diagnostic protocols, nucleic acid testing is suggested for acute infection, nasopharyngeal (NP), oropharyngeal (OP) swabs, wash in ambulatory patients, sputum, endotracheal aspirate, bronchoalveolar lavage samples are used for RT-PCR 25,26.
Surfaces Prone for SARS-CoV-2
Diverse material surfaces serves as the prone sites for persistence and onward transmission of SARS-CoV-2 from hours to 9 days. Persistence of virus over material surfaces varies with time depending on the temperature, humidity and material characteristics of surface. SARS-CoV-2 survives on material surfaces over long periods with simultaneous reduction in their persistence above 40oC 27,28. Survival of SARS-CoV-2 in different environmental media, including water, particulate matter, dust, and sewage under a variety of environmental parameters warrants their immediate systematic investigations 29. Subsequent studies confirm that SARS-CoV-2 remain infectious in water and sewage for days to weeks 30. Fig. 2 presents the quantitative persistence of CoVs on selected surfaces of material based structures remaining social contacts 26-31. In general, persistence of CoVs is observed on steel, 8h to 28 days 26-27, ceramics 28, aluminium 29, metals, alloys, paper and Teflon 30 over 5 days, rubber, neoprene, 8h, plastics, PVC and melamine over 48h to 5 days at 30oC 31. The simple house-keeping through periodic cleaning with commercially available virucidal agents may disinfect the material surfaces that temporarily restrict the transmittance of CoVs (Table 2). Alternative approaches on development of antiviral coatings, plastics, sprays, films and laminates are under active investigations by a range of manufacturers across the world.
 |
Figure 2: Persistence of SARS-CoV-2 and other CoVs on various inanimate surfaces. |
Table 2: Inactivation of SARS-CoV-2 using different virucidal agents
|
S.N. |
Virucidal agent |
Percent composition |
Virus |
Exposure time |
Reduction in viral infection |
Ref |
|
1 |
Ethanol |
95% 80% |
SARS- CoV MERS – CoV |
30s 30s |
5.5 4.0 |
32 33 |
|
2 |
2- Propanol |
100% 75% |
SARS- CoV MERS – CoV |
30s 30s |
3.3 4.0 |
34 33 |
|
3 |
1- Propanol |
0.2% |
SARS- CoV |
30s |
2.8 |
34 |
|
4 |
Formaldehyde |
1% |
SARS- CoV |
2 min |
3.0 |
34 |
|
5 |
Hydrogen peroxide |
0.5% |
H-CoV |
1 min |
4.0 |
35 |
Alcohol-based hand sanitizers (ABHS) containing isopropanol, ethanol, n-propanol (60% to 95%) due to their inherent virucidal activity assist the momentary reduction of infections against CoV 28 (Table 2). ABHS are effective against viral pathogens, such as Ebola virus, Zika virus (ZIKV), MERS-COV and SARS-CoV 31-34.
Antiviral Remedies
Herbal, homoeopathic and allopathic medications are merely the immune boosters and innovative vaccines are the only options to fight against the COVID-19 infection. Currently, there is no precise treatment except innovation of proper antiviral remedies against COVID-19. Antiviral vaccines working against SARS-CoV-2 are at the stage of active investigations subject to approval by WHO 31. To remain protected from SARS-CoV-2, it is essential to follow the preventive measures along with medication and vaccination and about 80% of COVID-19 cases itself get recover without needing any special treatment. Domestic activities through periodic home quarantine, hand cleaning, masking mouth and nose, drinking of lukewarm water (5L/day), steam inhalation, sanitizing the environment thorough washing of food stuffs, are the preliminary measures of prevention against WHO estimates vaccines may be available by March 2021.
Drugs and Intensive Care Treatment
A special emergency program for possible therapies SARS-CoV-2 is Coronavirus Treatment Acceleration Program (CTAP) has been constituted with the notion, to employ every available method to facilitate novel treatments to patients as quickly as possible 35. More than 350 clinical trials so far have been reviewed and at-least five distinct treatment plans have been authorized by FDA for emergency use against COVID-19 disease symptoms with varying levels of evidences. Drugs repurposing of already existing drugs on known targets offers a more rapid hope of tackling SARS-CoV-2. As the details of chemical steps of synthesis, toxicological and extensive clinical studies data are available, the repurposing of the known drugs is indeed an amicable strategy to combat the disease progression to a life threatening stage. Based on the extensive medicinal chemistry literature and clinical trial reports, five classes of repurposed drugs have been distinguished eliciting therapeutic responses against COVID-19 infection: as antiviral and antiphrastic, small molecules immune modulators, protease inhibitors, biologics for rheumatoid arthritis and kinase inhibitors 36.
Antiviral and Antiphrastic Drugs
Most antiviral drugs target major structural proteins of SARS-CoV-2, viz; RNA polymerase and few investigational drugs (Fig. 3) target fusion spike protein (S2 protein).
The Broad-Spectrum Antiviral Agent
Remdesivir
Remdesivir programs the insertion of three or five additional nucleotides at the end of the newly released RNA strand and thus delays the termination of RNA polymerization through frame shifting.
Study included a total of 1062 hospitalized patients with advanced COVID-19 and randomized with patients having lungs involvement. Results showed that patients treated with remdesivir recovered with 31% faster time of recovery than similar patients who received placebo (for remdesivir group on a median 11 days of recovery vs. 18 days for the placebo; p<0.001). Finally, a statistically non-significant difference was observed for mortality by day 15 (remdesivir 6.7% vs placebo 11.9%) or by day 29 (remdesivir 11.4% vs placebo 15.2%). The Lancet, however, reported the overall insignificant statistics of clinical benefits of remdesivir accounting for the overall frequency of mild to moderate adverse events (66% of patients getting intravenous remdesivir vs. 64% receiving a placebo developed adverse effects). Although, several groups involved with earlier NIH based clinical trials have raised strong 37.
Lopinavir–Ritonavir
Lopinavir–Ritonavir is a combination medicine used as a primary measure to prevent from HIV/AIDS infection after a known exposure or with other antiretroviral to treat HIV positive patients (Highly active antiretroviral therapy, HAART regimen). It has been proposed as a treatment for COVID-19 on the basis of in vitro activity, preclinical studies, and observational studies. The RECOVERY trial conducted during March to June-2020 had incorporated 176 hospitals in the UK. The design of this trial was an open-label controlled and randomized platform that accommodated 5040 COVID-19 patients who were randomly segregated into two groups. The group receiving standard of care (WHO) treatment had comprised twice the number of patients that collectively received the active treatment plan (either with Lopinavir-Ritonavir or with any other trial recommended plan as per patient’s eligibility) 38.
Ribavirin
Ribavirin (an oral Hepatitis-C antiviral) is a nucleoside inhibitor and terminates RNA synthesis via capping of growing mRNA chain with its guanosine like skeleton. There was no statistically significant differences in the clinical characteristics (ESR, CRP, TLC, DLC, serum creatinine, cough, dyspnea, infected lungs lobes number etc.) or support measures (invasive ventilation, Ig-therapy etc.) between groups receiving Ribavirin (500 mg OD) vs controls 39.
Ivermectin
Ivermectin is an endectocide antiparasitic drug mostly used against ascariasis, lymphatic filariasis, scabies or river blindness etc. Based on several in vitro studies using cell lines for nuclear protein, importin α/β1 heterodimer, it was also found to inhibit the translocation of various viral species proteins (SV40 protein of Simian virus), essentially required for their replication and viral capsid formation. Accordingly, this mechanism of inhibition of translocation through nucleus had earlier been found to effectively control a number of RNA viruses, such as West Nile virus, Dengue virus and Influenza virus etc. Its FDA approved dosage regimen is 0.2 mg/Kg of body weight one time that in more severe cases of filariasis or scabies may be repeated at least with two weeks spacing. The single dose corresponds to 9 mg (35 Kg to 50 Kg) to 12 mg for a normal adult of (51 to 65 Kg weight). Although the results of an in vitro study on SARS-COV-2 clinical isolates held in Australia, could not be translated to human infections and the investigators were not convinced with high dosage eliciting clinically relevant response, most of the developing south Asian countries have used this drug at excessive doses. However, if permitted, serious concerns of neurotoxicity and serious drug interactions with potent co-administered drugs may warrant careful considerations particularly when the patient is at risk of developing severe lung inflammations 40
Hydroxychloroquine and Chloroquine (HCQ & CQ)
Hydroxychloroquine and Chloroquine (HCQ & CQ) both are antimalarial and antirheumatic drugs HCQ/CQ have diversified mechanism of action that include (i) the interference in the endocytic pathway as lysosomotropic agent (increasing the pH of lysosomes and not allowing the transmembrane trafficking of viral proteins, phagolysosomal fusion and further modifications finally premature cleavage of S-protein before endocytosis); (ii) HCQ mediated inhibition of the processing of antigen over antigen presenting cells and owing to receptor affinity of APC towards immune complex, the down regulation of T-cells and B-cells and associated cytokines render the events of cytokine storm under control. In one clinical trial conducted in Europe, although nonrandomized, have shown a significant decline in the viral load during 10 days period of HCQ treatment 41.
Nitazoxanide
Nitazoxanide is an effective antiprotozoal, antihelmintic as well acts against a spectrum of parasitic infections and later it was found to inhibit replication of a broad range of respiratory viruses in cell cultures, including SARS-CoV-2. The two different phase 3 trials against COVID-19 were initiated in June 26, 2020 in high-risk populations, including elderly residents and healthcare workers 42.
Umifenovir (Arbidol)
Umifenovir (Arbidol) is used to treat influenza and acts by binding to haemagglutinin protein. Molecular dynamics and structural analysis studies suggested that Arbidol may have efficacy to treat COVID-19 that it will help in the development of new therapeutics for SARS-CoV-2 43.
Interleukin (IL)
Interleukin (IL) inhibitors are known to ameliorate several rheumatological diseases caused by autoimmune dysfunctioning mediated via the release of several cytokines and interferons. Oxygenation and incidence of inflammatory biomarker parameters also improved viz; high-sensitivity C-reactive protein, IL-6 etc. Structures of some important investigational repurposed drugs that may have potential anti-COVID-19 activity are presented in Fig. 3 44.
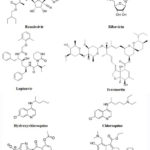 |
Figure 3: Structure of some Potential therapeutic COVID-19 drugs. |
Non-steroidal anti-inflammatory drugs
Non-steroidal anti-inflammatory drugs have recentlyobserved to eradicate COVID-19 through increased expression of angiotensin-converting enzyme 2 (ACE2) 45.
Plasma therapy
Plasma therapy has emerged as another therapeutic option in the struggle towards eradication of COVID-19. This therapy involvesadministration of COVID-19 convalescent plasma (CCP) donated by individuals having recovered from the disease is a prophylactic 46.
Complementary and Alternative Medicines
Herbal remedies are used by traditionally rich society to treat ailments naturally. A great philosopher, physician, botanist, and astrologer Philippus Aureolus Theophrastus Bombastus von Hohenheim (Paracelsus) had pointed out “all that human needs for health and wellbeing is available in nature, the challenge of science is to find it”. Herbs are the nature’s gift to human being that works as an alternative medicines to make disease free life. Practically 40% of medications utilized at present are either prompt plant segregates or chemically altered natural products 47. They are extremely rich in phytochemicals and have antimicrobial, antihypertensive, antidiabetic, cell fortification, hepatoprotective, cardioprotective, and other therapeutic applications 48 related to phytochemicals). In herbal several treatment options are available for enhancing immunity against respiratory illnesses like, Tinospora cordifolia, Osimum tenuiflorum, Piper nigrum, Piper longum, Withania somnifera, Curcuma longa, Elletaria cardamomum and citrus fruits (Fig. 4).
 |
Figure 4: Schematic diagram with different protective measures and recently |
Glycyrrhiza glabra commonly known in India with the name “Mulethi”, is used traditionally to treat coughs, colds, and disturbed digestion. It contains a triterpenoid glycyrrhizin also known as glycyrrhizic acid (GLR). Its amine salt diammonium glycyrrhizinate is an anti-inflammatory and anti-hepatitis B agent49. Clinical data showed that, patient treatment with diammonium glycyrrhizinate it is helpful in the recovery of a COVID-1950. GLR has a membrane destabilizing effect and the combination of GLR with natural products such as cyclodextrin and sterols may further inhibit the entry of the COVID-19 51.
Angiotensin-converting enzyme 2 (ACE2) functions as a SARS-CoV-2 receptor and GLR has a binding affinity with ACE2, which could stop the diffusion of the virus from the infected cell to the normal cell 52. Quinine an antimalarial medicine is isolated from the bark of the cinchona tree (Cinchona officinalis), its derived CQ and HCQ showed in vitro inhibition of COVID-19. Artemisinin is an antimalarial drug isolated from the herb sweet wormwood Artemisia annua employed in Chinese traditional medicine53. Chemically artemisinin is a sesquiterpene having internal an unusual peroxide bridge responsible its mechanism and action54. COVID-19 infections prompts release of Interleukin‐1 leads pulmonary fibrosis 55. Most of the pulmonary diseases are associated with oxidative stress. A. annua extract contains high phenolic content, which has significant antioxidant properties56,.57.
Table 3: Herbal extract having anti-viral activity related to COVID-19.
|
S.N. |
Active Compound |
Distribution (Plant) |
Mode of Action (Inhibition) |
Viral Activity |
Ref |
|
1 |
Quercetin |
Widely distributed |
Replication |
SARS-CoV |
58 |
|
2 |
Saikosaponins |
Bupleuri Radix |
Attachment, penetration |
CoVs-229E |
59, 60 |
|
3 |
Vitamin C |
Citrus fruits |
Immune Booster |
COVID-19 |
47 |
|
4 |
Terpenes (40) |
Ginseng |
Replication |
SARS-CoV |
61 |
|
5 |
Theaflavin-3,3′-digallate (TF3) |
Tea leaves (Camellia sinensis) |
3C-like protease (3CL(Pro)) and replication |
SARS-CoV |
62 |
|
6 |
Resveratrol |
Grapes, berries |
Replication |
MERS-CoV |
63 |
Strategic Vaccination and Immunization
As the current world is on the way to live with pandemic, there has been the growing need of search and accelerated deployment of safe and effective vaccines against COVID-19 for eventual solution to the public health crisis and immunological achievements. The current pandemic has endorsed as the leading cause of death in developing countries because the lack of appropriate vaccines. This is essential to ensure the coordinated approach for deployment of the vaccines and safe vaccination strategies. European center of disease prevention and control reveals clinical trials of around 19 vaccine candidates since 31 July 202064. European Medical Agency (EMA) has been under discussion with innovators of 38 potential COVID-19 vaccines and relies on their approval and deployment by the beginning of 202165.
Soon after the revelation of the genetic sequence of SARS-CoV-2, the coronavirus, on 11 January 2020, an intense impetus has mobilized the global R&D activity to develop a vaccine against the disease. The first COVID-19 vaccine candidate entered human clinical testing with unparalleled rapidity on 16 March 2020 employing next generation vaccine technology accelerated programs after proper economic assessment of the impact of COVID-19 pandemic. A copious contribution of the Coalition for Epidemic Preparedness Innovations to WHO authorities and vaccine developers to support the development of vaccines against COVID-19 is largely appreciated66.
On the basis of the detailed structure of the spike protein receptor binding domains (RBD) and information gained from preclinical studies with SARS-CoV and MERS-CoV, the spike protein was identified as an antigenic target for the development of a vaccine against SARS-CoV-2 at a very early stage. As contrary to the traditional Vaccine development, the process for SARS-CoV-2 has followed an accelerated timeline. Prior knowledge gained and with detailed Proteomic datasets, in this case the discovery phase was omitted. Existing processes were then followed and soon the interim results of phase I/II trials were used in Phase III trials with several clinical trial stages still running in parallel. Although it is yet not clear that how FDA policies like, emergency use authorization, would facilitate the licensing process to acquire biologics license applications, however, vaccine manufacturing companies have been undertaking risky ventures of the large-scale production of several vaccine candidates67.
The number of vaccine candidates is growing rapidly and based on many different strategic platforms, about 180 vaccine candidates are currently in development against COVID-19 SARS. The WHO has been rigorously maintaining the integrated and authenticated working documents that include most of the vaccines in development68. The platforms can be divided into ‘traditional’ approaches (inactivated or live-virus vaccines), platforms that have recently resulted in licensed vaccines (recombinant protein vaccines and vectored vaccines), and platforms that have yet to result in a licensed vaccine (RNA and DNA vaccines). Current SARS-CoV-2 vaccine candidates includes inactivated virus vaccines, live attenuated vaccines, recombinant protein vaccines based on the spike protein, the RBD or on virus-like particles, replication-incompetent vector vaccines and replication-competent vector vaccines, inactivated virus vector vaccines that display the spike protein on their surface, DNA vaccines and RNA vaccines. A special interest is derived from Replication-incompetent vectors which have culminated into a largest group of vaccines in development. Such vaccines are typically based on another virus that has been engineered to express the spike protein and has been disabled from replication in vivo by the deletion of parts of its genome. The majority of these approaches are based on adenovirus (AdV) vectors and these vectors are delivered intramuscularly. The vaccinated individuals then express the spike protein, to which the host immune system responds. Several replication-incompetent vector vaccine candidates against SARS-CoV-2 have progressed far in clinical development. Table 4 indicate an overview of some developed SARS-CoV-2 vaccines69
Table 4: An overview of some important SARS-CoV-2 developed vaccines
|
Company |
Vaccine candidate |
Dose range |
|
Sinovac |
PiCoVacc (inactivated virion+ aluminium hydroxide) |
3-6 µg |
|
Beijing Institute of Biological Products |
BBIBP- CorV (inactivated virion + aluminium hydroxide) |
4-8 µg |
|
AstraZeneca |
ChAdOxnCoV-19 (non- replicating AdV) |
2.4 ×1010 VP; 1× or 2 × (i.m.) |
|
Janssen |
Ad26COVS1 (non- replicating AdV) |
1 × 1011 VP (i.m.) |
|
Moderna |
mRNA-1273 (mRNA via LNPs) |
2 × 10-100 µg (i.m.) |
|
Novavax |
NVXCoV2373 (spike protein + Matrix- M) |
2 2.5- 25 µg |
Conclusions
Present century has witnessed the global spread of zoonotic CoVs originated from animals and its onward transmission to society. COVID-19 is the most recent pandemic of the present century threatening the lives of millions of people across the word. Transmission of SARS-CoV-2 was globally accelerated around February 2020 that has bound the society across many part of the world into home quarantine by March 2020. Control over infestation, propagation and onward cure of COVID-19 are still under constrains. The accelerated infestation has bound the society to think over the origin, symptoms, transmission, persistence and possible ways of diagnosis of COVID-19. Preventive measures through implications of antiviral remedies, intensive care treatment based on sanitizers, allopathic and alternative medicines, strategic vaccination and immunization are essential to remediate SARS-CoV-2. Presently society is struggling to face the situation of pandemic and only relying on human activities based on social distancing, sanization, masking and domestic remedies. Limited number of antiviral (Remdesivir, lopinavir–ritonavir, ribavirin, ivermectin), antimalarial (hydroxychloroquine , chloroquine) drugs, herbal extracts along with developed vaccination and immunization approved by WHO and FDA to remediate the pandemic of COVID-19.
Acknowledgement
The early financial support of UGC vide Ref No34-329/2008 SR/01-02-2009 funded to Department of Chemistry, G.B. Pant University of Agriculture & Technology Uttarakhand, is hereby acknowledged.
Conflict of interest
The author(s) declare(s) that there is no conflict of interests regarding the publication of this article.
References
- Leary, J.O; Kennedy, M.M; McGee, J.O. Mol Pathol. 1997; 50(1), 4-8
CrossRef - Malathi, V.G; Devi,P. R. Virusdisease. 2019; 30 (1), 3-12
CrossRef - Pelkonen, P.M; Tarvainen, K; Hynninen, A; Kallio, E.R; Henttonen, K; Palva, A; Vapalahti, O. Emerg. Infect. Dis. 2003; 9,1458- 1461
CrossRef - Lodish, H; Berk, A; Zipursky, S.L; Matsudaira, P; Baltimore, D; Darnell, J. Mol. Cell Bio. 4th ed 2000
- Zhu, N; Zhang, D; Wang, W; Li, X; Yang, B. N Engl J Med. 2020; 382 ,727–733
CrossRef - Kannan, S; Ali, P.S.S; Sheeza, A; Hemalatha, K. Eur. Rev. Med. Pharmacol. Sci. 2020; 24,2006-2011
- Zhu, N; Zhang, D; Wang, W; Li, X; Yang, B; Song, J; Niu, P. N. Engl. J. Med. 2020; 382,727-733
CrossRef - Wang, X; Xia, S; Wang, Q; Xu, W; Li, W; Lu, L; Jiang, S. Int. J. Mol. Sci. 2020; 21(11), 3843
CrossRef - Lv, L; Li, G; Chen, J; Liang, X; Li, Y. Front. Microbiol. 2020; 11, 3013.
CrossRef - Peeri, N.C; Shrestha, N; Rahman, M.S; Zaki, R; Tan, Z; Bibi, S; Haque, U; Int. J. Epidemiol. 2020; 49(3), 717–726
CrossRef - Wu, D; Wu, T; Liu, Q; Yang, Z. Int. J. Infect. Dis 2020; 94, 44-48
CrossRef - Ye, Z.W; Yuan, S; Yuen, K.S; Fung, K.S; Chan, C.P; Jin, D.Y; Int. J. Biol. Sci. 2020; 16(10) ,1686-1697
CrossRef - Giri, R; Bhardwaj, T; Shegane, M; Gehi, B.R; Kumar, P; Gadhave, K; Uversky,V.N; Cell Mol. Life Sci. 2020; 4, 1-34
- Cavanagh, D. Avian Pathol. 2005; 34(6), 439-448
CrossRef - Shi, J; Wen, Z; Zhong, G; Yang, H; Wang, C; Huang, B; Zhao, Y; Sci. 2020; 368, 1016-1020
CrossRef - Domańska-Blicharz, K; Woźniakowski, G; Konopka, B; Niemczuk, K; Welz, M; Rola, J; Cuvelier-Mizak, B. J. Vet. Res. 2020; 64(3), 333-345
CrossRef - Sun, J; He, Wang W.T; Lai, A; Ji, X; Zhai, X; Veit, M. Trends Mol. Med. 2020; 26(5),483-495
CrossRef - Guo, Y.R; Cao, Q.D; Hong, Z.S; Tan, Y.Y; Chen, S.D; Jin, H.J; Yan, Y. Mil. Med. Res.2020; 7(1),1-10
CrossRef - Chan, J.F; Kok, K.H; Zhu, Z. Emerg Microbes Infect. 2020; 9(1), 221-236
CrossRef - Lin, M; Beliavsky, A; Katz, K; Powis, J.E; Williams, V; Johnstone, J. Can. Med. Assoc. J. 2020; 192(12), 314-318
CrossRef - Sizun, J; Yu, M.W.N; Talbot, P.J. J. Hosp. Infect. 2000; 46(1), 55-60
CrossRef - Xu, J; Zhao, S; Teng,T, Abdalla, A.E; Zhu, W; Xie, L; Guo, X. Viruses. 2020; 12(2), 244
CrossRef - Weiss, S.R; Leibowitz, J.L. Adv Virus Res. 2011; 81, 85-164
CrossRef - Van, K; Doremalen, N; Bushmaker, T; Munster, V.J. Eur.surveill. 2013; 18 (38), 20590
CrossRef - Casanova, L.M; Jeon, S; Rutala, W.A; Weber, D.J; Sobsey, M.D. Appl. Environ. Microbiol. 2010; 76, 271
CrossRef - Warnes, S. L; Little, Z.R; Keevil, C.W. M. Bio. 2015; 6(6)
CrossRef - Duan, S.M; Zhao, X.S; Wen, R.F; Huang, J.J; Zhang, S.X. Biomed Environ Sci. 2003; 16, 246
- Lai, M.Y; Cheng, P.K; Lim, W.W; Clin. Infect. Dis. 2005; 41(7), 67-71
- Gostic, K; Gomez, A.C; Mummah, R.O; Kucharski, A.J; Smith, J.O. Elife2020; 9 ,55570
CrossRef - Venter, M; Richter, K. J Clin Pathol. 2020; 73, 370–377
CrossRef - Zeng, X; Song, X; Ma, T; Pan, X; Zhou, Y; Hou, Y; Cheng, F. J. Proteome. Res. 2020; 19(11), 4624-4636
CrossRef - Wang, Y; Zhang, D; Du, G; Du, R; Zhao, J; Yang, J. Lancet.2020; 395, 1569–78
- Franci, G; Falanga, A; Galdiero, S; Palomba, L; Rai, M; Morelli, G; Galdiero, M; Molecules. 2015; 20(5), 8856-8874
CrossRef - Dung, T.T.N; Nam, V.N; Nhan, T.T; Ngoc , T.T.B; Minh, L.Q; Nga, B.T.T; Quang, D.V. Mater. Res. Exp. 2020; 6(12) ,1250
CrossRef - Luo, H; Tang, Q.L; Shang, Y.X; Liang, S.B; Yang, M; Robinson, N; Liu, J.P. Chin. J. Integr. Med. 2020; 26(4), 243-250
CrossRef - Scavone, C; Brusco, S; Bertini, M; Sportiello, L; Rafaniello, C; Zoccoli, A; Capuano, A. Br. J. Pharmacol. 2020; 177(21), 4813-4824
CrossRef - Hazafa, A; Ur-Rahman, K; Haq, I.U; Jahan, N; Mumtaz, M; Farman, M; Bano, S. Drug. Metaob. Rev.2020; 52(3), 408-424
CrossRef - Cao, B; Wang, Y; Wen, D; Liu, W; Wang, J; Fan, G; Li, X. New Eng. J. Med. 2020; 382, 1787-1799
CrossRef - Khalili, J.S; Zhu, H; Mak, N.S.A; Yan, Y; Zhu, Y. J. Med. Virol. 2020; 92(7), 740-746
CrossRef - Gupta, D; Sahoo, A.K; Singh, A., Braz. J. Infect. Dis. 2020; 24(4), 369-371
CrossRef - Gautret, P; Lagier, J.C; Parola, P; Meddeb, L; Mailhe, M; Doudier, B; Honore, S. Int. J. Antimicrob. Agents 2020; 56(1), 105949
CrossRef - Mahmoud, D.B; Shitu, Z; Mostafa, A. J. Genet. Eng. Biotechnol. 2020; 18(1), 1-10
CrossRef - Che, J; Lin, S; Niu, C; Xiao, Q. Expert Rev. Respir. Med. 2020; 2, 1-9
- McGonagle, D; Sharif, K; O’Regan, A; Bridgewood, C. Autoimm. Rev. 2020; 19(6), 102537
CrossRef - Shanthanna, H; Cohen, S.P; Strand, H; Lobo, C.A; Eldabe, S; Bhatia, A; Narouze, S. Reg. Anesth. Pain Med. 2020; 2(1), 231
- Joyner, M.J; Wright, R.S; Fairweather, D; Senefeld, J.W; Bruno, K.A; Klassen, S.A; Rea, R.F. J. Clin. Investig. 2020; 11, 140200
- Veeresham, C. J. Adv. Pharm. Technol. Res. 2012; 3(4), 200-201
CrossRef - Venugopalan, S.K; Visweswaran, N. 2013; 3(7), 505-514
- Utsunomiya, H; Ichinose, M; Uozaki, M; Tsujimoto, K; Yamasaki, H; Koyama, A.H. Food. Chem. Toxicol. 2008; 46(6), 1919- 24
CrossRef - Ding, H; Deng, W; Ding, L; Ye, X; Yin, S; Huang, W. J Med Virol . 2020; 92(10), 2200-2204
CrossRef - Bin, M.D; Yeming, M.D; Wang, Danning; M.S. N. Engl. J. Med. 2020; 382, 1787-1799
- Luo, P; Liu, D; Li, J. Int. J. Antimicrob. Agents. 2020; 55, 105995
CrossRef - Gautret, P; Lagier, J.C; Parola, P; Hoang, V.T; Meddeb, L; Mailhe, M; Raoult, D. Int. J. Antimicrob. Agents. 2020; 56(1), 105949
CrossRef - Li, S.Y; Chen, C; Zhang, H.Q; Guo, H.Y; Wang, H; Wang, L; Li, R.S. Antivir. Res. 2005; 67(1), 18–23
CrossRef - Conti, P; Gallenga, C.E; Tete, G; Caraffa, A; Ronconi, G; Younes, A; Kritas, S.K. J. Biol. Regul. Homeost. Agents. 2020; 34(2), 11–16
- Ferreira, J.F; Luthria, D.L; Sasaki, T; Heyerick, A. Molecules. 2010; 15(5), 3135–3170
CrossRef - Enmozhi, S.K; Raja, K; Sebastine, I; Joseph, J. J Biomol Struct Dyn. 2020; 2(1), 1-7
CrossRef - Yi, L; Li, Z; Yuan, K; Qu, X; Chen, J; Wang, G. J. Virol. 2004; 78(20), 11334–11339
CrossRef - Cheng, P.W; Chiang, L.C; Lin, C.C. Clin. Exp. Pharmacol. Physiol. 2006; 33(7), 612–616
CrossRef - Guner,H.R; Hasanoglu, I; Aktaş, F. J. Med. Sci. 2020; 50, 571-577
CrossRef - Wu, C.Y; Jan, J.T; Ma, S.H; Kuo, C.J; Juan, H.F. Proc. Natl. Acad. Sci. 2004; 101(27), 10012–10017
CrossRef - Chen, C.N; Lin, C.P.C; Huang, K.K.; Chen, W.C; Hsieh, H.P; Liang, P.H. Alternat. Med. 2005; 2(2), 209–215
CrossRef - Lin, S.C; Ho, C.T; Chuo, W.H; Li, S; Wang, T.T; Lin, C.C. BMC Infect. Dis. 2017; 17(1), 144
CrossRef - Folegatti, P.M; Ewer, K.J; Aley, P.K; Angus, B; Becker, S; Belij-Rammerstorfer, S; Dold, C. The Lancet. 2020; 396(10249), 467-478
- Lurie, N; Saville, M; Hatchett, R; Halton, J. New Eng. J.Med. 2020; 382(21), 1969-1973
CrossRef - Krammer, F. Nature. 2020; 586(7830), 516-527.
CrossRef - Pallesen, J; Wang, N; Corbett, K. S; Wrapp, D; Kirchdoerfer, R. N; Turner, H. L; McLellan, J. S. Proc. Natl. Acad. Sci. 2017; 114 (35), 7348-7357.
CrossRef - Doshi, P. 2020; bmj, 371.
- Zhu, F. C; Li, Y. H; Guan, X. H; Hou, L. H;Wang, W. J; Li, J. X; Chen, W. Safety The Lancet. 2020; 395(10240), 1845-1854.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









