Aurantiol Schiff Base as a Raw Material in Fragrance Industry Synthesized by Simple Condensation Method and Its Characterization Using GC-MS
Candra Irawan1, Dian Islamiyati1, Andita Utami1, Imalia Dwi Putri2, Rika Perdana Putri3 and Singgih Wibowo1
1Departement of Chemical Analysis, Politeknik AKA Bogor, Bogor 16154, Indonesia
2Departement of Food Industry Quality Assurance, Politeknik AKA Bogor, Bogor 16154, Indonesia
3PT. Nilam Widuri Bogor, Indonesia
DOI : http://dx.doi.org/10.13005/ojc/360332
Article Received on : 06 May 2020
Article Accepted on : 07 Jun 2020
Article Published : 12 Jun 2020
Fragrance seeds are produced from a combination of various kinds of raw materials, both natural and synthetic. One of the synthetic raw materials added in making fragrances is schiff base. In this study, aurantiol schiff base was made from raw material of methyl anthranylate and hydroxy citronellal by simple condensation at 110 °C for 30 minutes. The color of the product obtained is observed and compared to the standard color, which is dark yellow. Furthermore, characterization was carried out using gas chromatography and mass spectrophotometry. The chromatogram results showed that aurantiol with the molecular formula C18H27NO3 had similarities above 90%.
KEYWORDS:Aurantiol; Condensation Method; Fragrance; GC-MS; Schiff Base
Download this article as:| Copy the following to cite this article: Irawan C, Islamiyati D, Utami A, Putri I. D, Putri R. P, Wibowo S. Aurantiol Schiff Base as a Raw Material in Fragrance Industry Synthesized by Simple Condensation Method and Its Characterization Using GC-MS. Orient J Chem 2020;36(3). |
| Copy the following to cite this URL: Irawan C, Islamiyati D, Utami A, Putri I. D, Putri R. P, Wibowo S. Aurantiol Schiff Base as a Raw Material in Fragrance Industry Synthesized by Simple Condensation Method and Its Characterization Using GC-MS. Orient J Chem 2020;36(3). Available from: https://bit.ly/3cREhnq |
Introduction
Schiff base (azomethine / anils / imines) which has azomethine (-CH = N-) group belongs to a group of compounds that have various biological activities such as anticancer, antimicrobial, antiviral, antitumor, antineoplastic, antitubercular, anti-HIV, anthelmintic, anticonvulsant, antimalarial, antiplatelet, antibiotic, diuretic, anti-inflammatory, and analgesic.1,2,3 In addition, schiff base is also often used in spices and fragrances because of its characteristic odor.4 Some schiff bases are reported to give the smell of orange flowers.5.6
Schiff base is a compound obtained from a condensation reaction between aldehydes and primary amines and produces water as a by-product.7,8 The amines which are commonly used in the fragrance prodcution are methyl anthranilate and ethyl anthranilate, while aldehydes commonly used in fragrances are alkyl aromatic or aldehyde, terpene aldehyde, such as hydroxycitronellal.9 There are many types of schiff base, such as lilyantine, jasmea, verdantiol,10 aurantiol,11 lyrame,12 cyclantine,13 and citral-methylantranylate.14 The most famous type of schiff base and widely used in various kinds of flower fragrance seeds are verdantiol, aurantiol, and lyrame.15
Aurantiol has the IUPAC name: methyl-N-3,7-dimethyl-7-hydroxyoctyliden anthranilate, and several trade names such as aurantiol, auriol, auranol, auralva, antralal, aurangeol, aurantine, and bigariol. Aurantiol has the molecular formula C18H27NO3, with a molecular weight of 305.43 g/mol.16 In the previous study, aurantiol was synthesized with simple condensation at a temperature of about 90 ± 5 °C with a time variation for 15, 30, 60, 120, 180, and 240 min. The results showed that the optimal synthesis time about 30 to 60 min can be used as a reference for aurantiol synthesis in an industrial scale.11 In this experiment, synthesis of aurantiol was carried out with simple condensation by increasing the temperature at 110 °C for 30 min. It is expected that by increasing the temperature can optimize the synthesis process.
Experimentals
Aurantiol schiff base was prepared by mixing methyl anthranylate solution and hydroxycitronellal solution. Then, the mixture was heated and stirred on a hotplate magnetic stirrer with a temperature of 110 °C for 30 min. The color of aurantiol schiff base was compared to the standard colors. In order to confirm the obtained aurantiol schiff base, Agilent 5975C Gas Chromatography – Mass Spectrometer was applied. The aurantiol schiff base could be detected from the mass spectra. Finally, fragmentation patterns were made to comfirm the obtained compounds. The analysis on condition of GC-MS can be seen at Table 1 below.12
Table 1: GC-MS Analysis Condition
|
|
Information |
|
Column |
Capillary Column : HP-5 (5% Phenyl Methyl Siloxane 325 °C |
|
Carrier Gas |
Helium |
|
Carrier Gas Pressure |
7.0531 psi |
|
Injection Technique |
Split |
|
Injector Temperature |
100 °C |
|
Injection Volume |
0.2 mL |
|
Split Ratio |
80 : 1 |
|
Temperature Program (Column) |
|
|
Initial Temperature |
100 °C hold for 5 min |
|
Temperature rate |
15 °C per min |
|
Final Temperature |
250 °C hold for 5 min |
|
Interval |
25 min |
Result and Discussion
The obtained aurantiol schiff base compared to standard product of PT Nilam Widuri is shown in Figure 1. The colour of aurantiol was dark yellow and the standard had a thicker color. The aurantiol schiff base is formed from hydroxycitronellal which has a carbonyl group. It reacts with the amine group in methyl anthranilate through the cross-aldol condensation reaction stage.
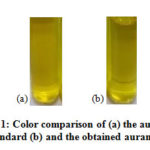 |
Figure 1: Color comparison of (a) the aurantiol standard (b) and the obtained aurantiol |
Table 2: The GC result of aurantiol schiff base
|
Retention Time (Min) |
Compounds |
Compotition (%) |
Molecular Formula |
|
8.680 |
Hydroxycitronellal |
20.68 |
C10H20O2 |
|
9.465 |
Methyl Anthranilate |
32.82 |
C8H9NO2 |
|
16.923 |
Aurantiol |
30.85 |
C18H27NO3 |
|
16.981 |
Aurantiol |
15.65 |
C18H27NO3 |
Table 2 shows aurantiol with the molecular formula C18H27NO3 (molecular weight = 305.42 gram/mol) and similarities above 90%. The aurantiol compound was identified at last, because the large molecular weight of aurantiol can cause the compound to be strongly bound to the stationary phase. Aurantiol boiling point is high (low volatility) of 241 °C, while the injector temperature is set at 100 °C and the maximum temperature of program column is 325 °C can cause the gas phase of aurantiol not to form homogeneously since it is in the inlet, so aurantiol is identified more than one peak with different time retention. From this result, it was shown that the product contained not only aurantiol schiff base, but it also contained hydroxycitronellal and methyl anthranylate. The mass spectrum of the aurantiol compound in the product can be seen in Figure 2.
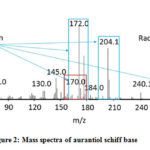 |
Figure 2: Mass spectra of aurantiol schiff base |
The mass spectrum in Figure 2 shows many peaks with different m/z values and the main peaks are at m/z value of 59.0; 170.0; 172.0; 204.1; 272.1; 287; and 305.1. The mass spectrum of compounds in Figure 2 shows the presence of a molecule (M+) of aurantiol schiff base (Methyl-N-3,7-dimethyl-7-hydroxyoctylidene anthranilate) at m/z = 305.1 which equals to its molecular weight about 305.42 g/mol. The highest peak at m/z of 172.0 is the base peak, the m/z value of 59.0 is the base showing the lowest peak among the others. The peak with an m/z value of 204.1 is a cation. The following figures show the illustration of molecular ion formation, base peaks, cation and cation radicals:
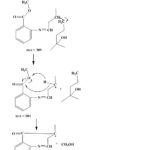 |
Figure 3: Fragmentation of aurantiol Schiff base |
Conclusion
The aurantiol schiff base was successfully synthesized by a simple condensation at 110 °C for 30 min. From the chromatogram results, 2 peaks were identified as aurantiol at retention times of 16.923 and 16.981 minutes. Based on mass spectrum, the molecular ion of aurantiol schiff base was detected at m/z value of 305.1.
Acknowledgements
We would like to thank Politeknik AKA Bogor to fully support our research and PT Nilam Widuri for helping us during the experiments.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- Villa, C.; Gambaro, R.; Mariani, E.; Dorato, Journal of Pharmaceutical and Biomedical Analysis. 2007,44,755-762.
CrossRef - Herman, S. Allured Publishing Corp. 2002. England.
- Ansari, R.M.; Bhat, B. J. Chem. Sci. 2017,129,1483–1490.
CrossRef - Da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Cleide, V.B.; Martins, C.V.B.; de Fatima, A. Adv.Research. 2011,2,1–8.
CrossRef - Nagesh, G.Y.; Mruthyunjayaswamy, B.H. J. Molecular Structure. 2015, 1085, 198-206.
CrossRef - Rowe, D. Blackwell Publishing Ltd. 2005. USA.
- Quellet, C.; Schudel, M.; Ringgenberg, Chimia. 2001, 55, 421-428.
- Gygax, H.; Koch, Chimia. 2001, 55,401-405.
- Calkin, R.R.; Jellinek, J.S. John wiley & Sons, Inc. 1994. Canada.
- Dhokale, N.T.; Karale, B.; Nagawade, A.V. Orient. J. Chem. 2017, 33(1),165-172.
CrossRef - Sarkic, A.; Stappen, I. Cosmetics. 2018, 5 (1),
CrossRef - Kumar, A. Departement Pharmacy University of Punjab Technical Jalandhar. 2011. India.
- Cortez-Pereira, C.S.; Baby, A.R.; Velasco, M.V.R. Cosmetic Dermatology. 2010,9, 230–241.
CrossRef - Sell, C. RSC Publishing. 2006. Dorchester.
- Irawan, C.; Indryati, S.; Lestari, E.S.; Hidaningrum, A.; Orient. J. Chem. 2018,34(1), 394-400.
CrossRef - Irawan, C.; Islamiyati, D.; Putri, R.P.; Madiabu, M.J.; Supriyono. J. Chem. 2018, 34,3118-3122.
CrossRef - Asten, A. TrAC-Trend Anal Chem. 2002, 21(9–10),698–708.
CrossRef - Begnaud, F.; Chaintreau, Phil. Trans. R. Soc. A, 2016,374,20150365.
CrossRef - Debonneville, C.; Chaintreau, J. Chromatogr. A, 2004, 1027, 109-115.
CrossRef - Harianingsih, R.; Wulandari, C.; Harliyanto, C.N.; Techno, 2017.18 (1),23–27.
- Fessenden, R.J.; Fessenden, J.S. Grant Press. 1982. Boston, USA.
- McLafferty, F.W. University science books. 1980.California, USA.
- Mishra, A.P; Khare, M; Gautam, S.K., & React. Inorg. Met-Org. Chem., 2002, 32, 1485-1500.
CrossRef - Amer, S.; Wakiel, N.E.; Ghamry, H.E., Mol. Str., 2013, 1049, 326– 335.
CrossRef - Ali, A.; Abdullah, N.; Maah, M.J., J. Chem., 2013, 25, 3105-3108.
CrossRef - Arctander, S. Perfume and flavor chemicals (Aroma chemicals). Vol. 2nd ; Allured Publishing Corporation, Illinois, USA, 1969.
- Baudin, J. Schiff bases, U. S. Patent 5,264,615, 1993.
- Blane, P. A.; Aschiero, R. Fragrance Ingredient, US Patent No. 5,155,095, 1992.
- Dhokale, N.T.; Karale, B.K.; Nagawade, A.V., J. Chem., 2017, 33(1), 165-172.
CrossRef - Raikwar, K.; Agarwal, D.D.; J. Chem., 2015, 31(1), 547-551.
CrossRef - Cortez-Pereira, C.S.; Baby, A.R.; Velasco, M.V.R., Cosmetic Dermatology, 2010, 9, 230–241.
CrossRef - Sell, C.S., The Chemistry Of Fragrances: From Perfumer To Consumer 2nd Edition, RSC Publishing, Dorchester, 2006.
- Irawan, C.; Indriyati, S.; Lestari, E.S.; Hidaningrum, A.; Supriyono, J. Chem., 2018, 34 (1), 394.
CrossRef - Irawan, C.; Islamiyati, D.; Putri, R.P.; Madiabu, M.J.; Supriyono, J. Chem., 2018, 34, 118.
CrossRef - Irawan, C.; Juhana, S.; Hanafi, Rochaeni, H.; Fajri, M.Y.; Putri, R.P., J. of Chem. Stud., 2017, 5(5), 475-479.
CrossRef - Irawan, C.; Nur, L.; Mellisani, B.; Arinzani; Hanafi, RASAYAN J.Chem., 2019, 12(2), 951-959.
CrossRef - Ohloff G., Scent and Fragrances, Springer-Verlag, Berlin, 1994.
CrossRef - Herman S.J., Fragrance Applications: A Survival Guide. Allured Publishing Corp., England, 2002.

This work is licensed under a Creative Commons Attribution 4.0 International License.









