Sebacoyl Isothiocyanate in the Synthesis of Bis (1,3,4-Thiadiazole, 1,3,4-Thiadiazolo[3,2-a]Pyridine, 4-Thiazolidinone, Chromenes, and Naphtho[1,2-b][1,4]Oxazine) Derivatives
Hamdy Khamees Thabet
Chemistry Department, Faculty of Arts and Science, Northern Border University, Rafha-91911, PO 840, Saudi Arabia.
Corresponding Author E-mail: hamdykhamees@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/360215
Article Received on : 12-Dec 2019
Article Accepted on :
Article Published : 30 Apr 2020
Reaction of sebacoyl isothiocyanate with cyanoacetic acid hydrazide afforded N1,N10-bis(1,3,4-thiadiazole) derivative which used as key precursor for the preparation of bis(1,3,4-thiadiazolo[3,2-a]pyridine, 4-thiazolidinone, chromene, and naphtho[1,2-b][1,4]oxazine) derivatives. The chemical structures of novel compounds were proven by spectroscopic analyses.
KEYWORDS:Chromenes; Naphtho[1,2-b][1,4]Oxazine; Sebacoyl Isothiocyanate; 1,3,4-Thiadiazoles; 1,3,4-Thiadiazolo[3,2-a]Pyridines; 4-Thiazolidinone
Download this article as:| Copy the following to cite this article: Thabet H. K. Sebacoyl Isothiocyanate in the Synthesis of Bis (1,3,4-Thiadiazole, 1,3,4-Thiadiazolo[3,2-a]Pyridine, 4-Thiazolidinone, Chromenes, and Naphtho[1,2-b][1,4]Oxazine) Derivatives. Orient J Chem 2020;36(2). |
| Copy the following to cite this URL: Thabet H. K. Sebacoyl Isothiocyanate in the Synthesis of Bis (1,3,4-Thiadiazole, 1,3,4-Thiadiazolo[3,2-a]Pyridine, 4-Thiazolidinone, Chromenes, and Naphtho[1,2-b][1,4]Oxazine) Derivatives. Orient J Chem 2020;36(2). Available from: https://bit.ly/3bQE2JD |
Introduction
Acyl isothiocyanates are predominant bioactive compounds having many biological activities and are versatile precursors to the preparation of sulfur-containing heterocycles1-7.
Among the wide range of heterocycles studied to develop pharmaceutically significant molecules, 1,3,4-thiadiazole has played a significant role in medicinal chemistry. Moreover, 1,3,4-thiadiazole nucleus has fascinated significance in medicinal chemistry, displaying pharmacological properties such as, antimicrobial8-10, anti-inflammatory11, antitumor12,13, anticonvulsant14,15, antioxidant16, antifungal17, antitubercular18, and antidepressant activities19.
In view of these results and in extension of our work on bioactive heterocycles20-28, we report herein the efficient preparation of bis (1,3,4-thiadiazolo[3,2-a]pyridine, 4-thiazolidinone, chromenes, and naphtho[1,2-b][1,4]oxazine) derivatives containing 1,3,4-thiadiazole starting from sebacoyl isothiocyanate as a key precursor.
Materials and Methods
The melting point, (1H/13C)-NMR, and elemental analysis data was obtained by the techniques described in our earlier reports20.
N1,N10-Bis(5-(Cyanomethylene)-4,5-Dihydro-1,3,4-Thiadiazol-2-yl)Decanediamide (4)
NH4SCN (1.52g, 0.02 mol) and sebacoyl chloride (2.39g, 0.01 mol) in dioxane (30 mL) was mixed, then the mixture was stirred for 30 minutes and the precipitate was filtered off. To the remainder filtrate 2-cyanoacetohydrazide (1.98g, 0.02 mol) in dioxane (15 mL) was added then refluxed for 5 hours. The reaction product was filtered, and left to dry, then crystallized to afford 4 (Table 1).
N1,N10-Bis(5-(1-Cyano-2-Methylprop-1-en-1-yl)-1,3,4-Thiadiazol-2-yl)Decanediamide (5)
NH4SCN (1.52g, 0.02 mol) and sebacoyl chloride (2.39g, 0.01 mol) in acetone (50 mL) was underwent stirring for thirty minutes at room temperature, and 2-cyanoacetohydrazide (1.98g, 0.02 mol) was added. The solution underwent refluxing for 6 hrs., cooled at the room temp. The reaction product was filtered, and left to dry, then crystallized to afford 5 (Table 1).
Synthesis of N1,N10-Bis(1,3,4-Thiadiazol-2-yl)Decanediamide (7a-c).
General method: A mixture of 4 (0.01 mol), aromatic aldehydes 6a-c (0.02 mol), and pip. (3 drops) in EtOH (25 mL) underwent refluxing for 6 hrs. The reaction product was filtered, and left to dry, then crystallized to afford 7a-c, (Table 1).
Synthesis of N1,N10-Bis(1,3,4-Thiadiazolo(3,2-a)Pyridine-2-yl)-Decanediamide (9a-c).
Method A
To compound 4 (0.01 mol) and requisite cinnamonitrile (0.02 mol) in EtOH (30 mL), two piperidine drops was mixed. The reaction mixture was refluxed for three hrs. The solution was cooled, the solid mass was filtered, then purified to afford 9a–c.
Method B
To a mixture of the requisite arylidene derivatives 7a-c (0.01 mol) and CH2(CN)2 (0.02 mol) a few drops of piperidine in EtOH (30 mL) was added and refluxed for 4 hrs. The formed precipitate was filtered, and then purified to afford 9a–c (Table 1).
N1,N10-Bis(5-(Cyano(2-Oxoindolin-3-Ylidene)Methylene)-1,3,4,Thiadiazol-2-yl)-Decanediamide (10)
To a compound 4 (0.446g, 1mmol) and isatin (0.294g, 2mmol) in EtOH (30 mL), three piperidine drops was added. The solution underwent refluxing for 2 hrs. The solid mass on hot, was collected, dried, finally recrystallized to afford 10 (Table 1).
N1,N10-Bis(5-(4-Amino-3-Phenyl-2-Thioxo-2,3-Dihydrothiazol-5-yl)-1,3,4-Thiadiazol-2-yl)Decanediamide (11)
A mixture of 4 (0.446g, 1mmol), phenyl isothiocyanate (0.27g, 2 mmol) and elemental sulfur (0.064g, 2 mmol) in dioxane (30 mL) having Et3N (1 ml) was allowed to reflux for 3hrs. The reaction product was filtered, then recrystallized to afford 11 (Table 1).
N1,N10-Bis(5-((4-oxo-4,5-Dihydrothiazol-2-yl)Methylene)-1,3,4-Thiadiazol-2-yl)Decanediamide (12)
A solution of 4 (0.446g, 1mmol), and sulfanylacetic acid (0.184g, 2mmol) in AcOH (20 mL) underwent refluxing for 4 hours. The solid was collected and purified to afford 12 (Table 1).
Synthesis of Bis-Chromene Derivatives (14), (16) and Bis(Naphtho[2,1-b][1,4]Oxazine Derivative (17): A mixture of 4 (0.446g, 1mmol), salicylaldehyde (0.244g, 2 mmol); 2-hydroxy-1-naphthaldehyde (0.344g, 2 mmol); 1-nitrosonaphthalen-2-ol (0.345g, 2mmol) and AcONH4 (2 g) in EtOH (30 mL) underwent refluxing for two hrs. The product mass was collected, washed with MeOH, dried, and finally purified to afford 14, 16 and 17 (Table 1).
Synthesis of N1,N10-Bis(5-(2-oxo-2H-Chromen-3-yl)-1,3,4-Thiadiazol-2-yl)-Decane-Diamide (15)
Method A
A mixture of 4 (0.446g, 1mmol), salicylaldehyde (0.244g, 2 mmol) and 0.5g of fused AcONa in AcOH (30 mL) underwent refluxing for 2 hrs. The formed product mass on cooling was collected, dried, and finally purified to afford 15, (Table 1).
Method B
To a refluxing solution of bis(iminochromene) derivative 14 (1.31g, 2mmol) in dioxane (30 mL) HCl (5 mL) was added. The refluxing was continuing for 2 hrs., and left to cool. The obtainable solid was collected, washed with cold H2O, dried, and finally crystallized to afford 15.
Table 1: Physical data of the compounds
|
Compd. No. |
M.P. (oC) |
Yield (%) Cryst. Solvent |
Formula (Mol. Wt.) |
Elemental Analyses Calcd. /Found % |
||
| C |
H |
N |
||||
|
4 |
220-22 |
80 (A) |
C18H22N8O2S2 (446.55) |
48.42 48.27 |
4.97 4.74 |
25.09 25.16 |
|
5 |
232-34 |
68 (B) |
C24H30N8O2S2 (526.68) |
54.73 54.52 |
5.74 5.49 |
21.28 21.10 |
|
7a |
245-47 |
66 (B) |
C32H30N8O2S2 (622.77) |
61.72 61.53 |
4.86 4.70 |
17.99 17.76 |
|
7b |
256-58 |
71 (B) |
C34H34N8O4S2 (682.82) |
59.81 59.65 |
5.02 4.89 |
16.41 16.28 |
|
7c |
264-66 |
80 (B) |
C32H28Cl2N8O2S2 (691.65) |
55.57 55.42 |
4.08 3.84 |
16.20 15.93 |
|
9a |
279-81 |
55/53 (B) |
C38H34N12O2S2 (754.89) |
60.46 60.31 |
4.54 4.40 |
22.27 22.08 |
|
9b |
284-86 |
80/74 (B) |
C40H38N12O4S2 (814.94) |
58.95 58.70 |
4.70 4.49 |
20.63 20.48 |
|
9c |
290-92 |
88/85 (B) |
C38H32Cl2N12O2S2 (823.78) |
55.41 55.25 |
3.92 3.71 |
20.40 20.25 |
|
10 |
275-77 |
81 (B) |
C34H28N10O4S2 (704.78) |
57.94 57.76 |
4.00 3.83 |
19.87 19.64 |
|
11 |
240-42 |
77 (B) |
C32H32N10O2S6 (781.04) |
49.21 49.10 |
4.13 3.96 |
17.93 17.71 |
|
12 |
263-65 |
74 (B) |
C22H26N8O4S4 (594.74) |
44.43 44.27 |
4.41 4.22 |
18.84 18.70 |
|
14 |
251-53 |
83 (B) |
C32H30N8O4S2 (654.76) |
58.70 58.52 |
4.62 4.45 |
17.11 16.89 |
|
15 |
285-87 |
75/60 (B) |
C32H28N6O6S2 (656.73) |
58.52 58.36 |
4.30 4.17 |
12.80 12.63 |
|
16 |
268-70 |
77 (B) |
C40H34N8O4S2 (754.88) |
63.64 63.51 |
4.54 4.39 |
14.84 14.67 |
|
17 |
289-91 |
80 (B) |
C38H32N10O4S2 (756.86) |
60.30 60.18 |
4.26 4.11 |
18.51 18.32 |
A = EtOH, B = dioxane
Table 2: The characteristic spectroscopic data of 4, 5, 7a-c, 9a-c, 10-12, and 14-17
|
Compound |
IR |
1H-NMR |
13C-NMR |
|
4 |
3205, 2927, 2851, 2263, 1685, 1609 |
1.26 (brs, 8H.), 1.57 (m, 4H.), 2.36 (t, 4H.), 3.74 (s, 2H), 10.13 (s, 2H, 2NH) |
18.73 (1C.), 25.06 (1C.), 28.93 (1C.), 29.04 (1C.), 34.10 (1C.), 116.94 (C≡N), 154.07, 167.32 (C=N), 172.20 (C=O) |
|
5 |
3209, 2926, 2851, 2252, 1691, 1609 |
1.26 (brs, 8H.), 1.56 (m, 4H), 2.37 (t, 4H.), 2.23 (s, 3H), 2.39 (s, 3H), 10.16 (s, 2H, 2NH) |
23.56 (1C), 24.03 (1C), 25.40 (1C.), 28.88 (1C.), 29.28 (1C.), 39.56 (1C.), 116.11 (C≡N), 158.49, 164.36 (C=N), 171.41 (C=O) |
|
7a |
3190, 3017, 2939, 2853, 2215, 1687, 1587 |
1.28 (brs, 8H.), 1.56 (m, 4H.), 2.36 (t, 4H.), 7.04-7.35 (m, 10H, Ar-H), 8.45 (s, 2H, benzylidene-H), 10.12 (s, 2H, 2NH) |
24.93 (1C.), 28.83 (1C.), 29.28 (1C.), 34.56 (1C.), 98.58 (C2), 116.11 (C≡N), 146.15 (benzylidene’C), 127.55, 128.05, 131.11, 136.42 (Ar’C), 158.49, 164.36 (C=N), 171.41 (C=O) |
|
7b |
3209, 3050, 2925, 2851, 2205, 1682, 1610 |
1.29 (brs, 8H.), 1.54 (m, 4H.), 2.38 (t, 4H.), 3.83 (s, 3H), 7.13-7.26 (m, 8H, Ar-H), 7.97 (s, 2H, benzylidene-H), 9.85 (s, 2H, 2NH) |
24.68 (1C), 28.89 (1C.), 29.32 (1C.), 34.35 (1C.), 56.06 (1C), 116.54 (C≡N), 99.64 (C2), 125.67, 129.77, 135.52, 142.16 (Ar’C), 147.60 (benzylidene’C), 158.70, 162.71 (C=N), 172.30 (C=O) |
|
7c |
3307, 3093, 2939, 2860, 2206, 1680, 1601 |
1.28 (brs, 8H), 1.60 (m, 4H), 2.38 (t, 4H), 7.38-7.46 (m, 8H), 8.44 (s, 2H, benzylidene-H), s 9.95 (s, 2H, 2NH) |
22.09 (1C.), 28.65 (1C.), 29.15 (1C.), 34.34 (1C.), 115.41 (C≡N), 94.06 (C2), 126.79, 127.43, 130.42, 133.48, 137.25 (Ar’C), 147.78 (benzylidene’C), 155.48, 163.56 (C=N), 173.21 (C=O) |
|
9a |
3327, 3201, 2931, 2865, 2210, 1682, 1605 |
1.26 (brs, 8H), 1.57 (m, 4H), 2.37 (t, 4H), 5.76 (s, 2H, pyridine H-4), 6.23 (br. s, 4H, 2NH2), 7.35-7.55 (m, 10H, Ar-H), 10.21 (s, 2H, 2NH) |
24.94 (1C.), 27.82 (1C.), 29.00 (1C.), 34.33 (1C.), 45.31 (C-7), 63.21 (C-6), 101.15 (C-8), 115.81, 116.30 (C≡N), 155.98 (C-5), 126.55, 128.62, 131.11, 136.42 (Ar’C), 151.49 (C-8a), 161.90 (C-2), 171.41 (C=O) |
|
9b |
3364, 3214, 2926, 2853, 2216, 1677, 1612 |
1.26 (brs, 8H), 1.56 (m, 4H), 2.38 (t, 4H), 3.78 (s, 3H), 5.83 (s, 2H, pyridine H-4), 6.31 (br.s, 4H, 2NH2), 7.19-7.32 (m, 8H, Ar-H), 10.05 (s, 2H, 2NH) |
25.02 (1C.), 27.65 (1C.), 28.45 (1C.), 34.16 (1C.), 46.71 (C-7), 64.58 (C-6), 100.83 (C-8), 115.20, 117.11 (C≡N), 156.44 (C-5), 126.49, 128.75, 131.28, 137.23 (Ar’C), 152.51 (C-8a), 160.82 (C-2), 172.34 (C=O) |
|
9c |
3331, 3256, 2918, 2847, 2224, 1680, 1605 |
1.27 (brs, 8H), 1.56 (m, 4H), 2.37 (t, 4H), 5.79 (s, 2H, pyridine H-4), 6.20 (brs, 4H, 2NH2), 7.25-7.41 (m, 8H, Ar-H), 10.16 (s, 2H, 2NH) |
25.15 (1C.), 27.41 (1C.), 28.90 (1C.), 33.89 (1C.), 47.25 (C-7), 65.18 (C-6), 99.74 (C-8), 115.55, 116.28 (C≡N), 155.21 (C-5), 127.35, 128.86, 130.26, 137.06 (Ar’C), 153.59 (C-8a), 161.15 (C-2), 171.37 (C=O) |
|
10 |
3208, 3173, 3053, 2925, 2852, 1700, 1676, 1609 |
1.28 (brs, 8H), 1.61 (m, 4H), 2.37 (t, 4H), 7.10-8.11 (m, 8H, Ar-H), 10.35, 11.51 (brs, 4H, 4NH) |
23.86 (1C.), 26.45 (1C.), 27.35 (1C.), 34.22 (1C.), 114.75 (C-C≡N), 117.21 (C≡N), 112.95, 124.36, 125.37, 126.24, 128.61, 142.74 (Ar’C), 154.65 (indoline-C3), 155.32, 162.44 (C-2, C-5), 169.81, 173.37 (C=O) |
|
11 |
3350, 3240, 3161, 3061, 2925, 2853, 1690, 1619 |
1.26 (brs, 8H), 1.55 (m, 4H), 2.39 (t, 4H), 6.58 (s, 4H, 2NH2), 7.34-7.63 (m, 10H, ArH), 10.10 (s, 2H, 2NH) |
24.92 (1C.), 25.38 (1C.), 28.67 (1C.), 34.19 (1C.), 83.35 (thiazolidine’C), 129.33, 129.40, 130.59, 130.63, 135.60 (Ar’C), 146.05 (thiazolidine’C), 154.86, 154.96 (C=N), 171.84 (C=O), 185.70 (C=S) |
|
12 |
3209, 3051, 2923, 2851, 1732, 1681, 1609 |
1.27 (brs, 8H), 1.59 (m, 4H), 2.38 (t, 4H), 3.84 (s, 2H), 4.13 (s, 2H), 10.15 (s, 2H, 2NH) |
24.14 (1C.), 25.40 (1C.), 28.89 (1C.), 34.33 (1C.), 39.96 (1C.), 45.63 (1C), 161.64, 163.02, 167.32 (3C=N), 171.98, 178.21 (2C=O) |
|
14 |
3283, 3135, 3021, 2928, 2854, 1678, 1637 |
1.29 (brs, 8H), 1.57 (m, 4H), 2.37 (t, 4H), 7.40-8.13 (m, 8H, Ar-H), 8.50 (s, 2H, chromene-H4), 9.16 (s, 2H, 2NH), 10.35 (s, 2H, 2NH) |
25.31 (1C.), 26.21 (1C.), 28.63 (1C.), 34.51 (1C.), 116.41, 118.71, 120.08, 127.64, 129.82, 130.93, 132.87, 153.45 (Ar’C), 155.67, 158.16 (C=N), 168.68 (C=NH), 171.94 (C=O) |
|
15 |
3225, 3133, 3042, 2944, 2861, 1740, 1694, 1616 |
1.28 (brs, 8H), 1.55 (m, 4H), 2.38 (t, 4H), 7.28-8.23 (brs, 8H, Ar-H), 8.63 (s, 2H, chromene-H4), 10.40 (s, 2H, 2NH) |
25.36 (1C.), 26.55 (1C.), 29.23 (1C.), 34.68 (1C.), 115.89, 119.28, 121.43, 127.82, 129.56, 130.14, 131.74, 153.63 (Ar’C), 156.31, 159.24 (C=N), 163.26, 169.57 (C=O) |
|
16 |
3277, 3158, 3044, 2921, 2835, 1682, 1614 |
1.28 (brs, 8H), 1.55(m, 4H), 2.37 (t, 4H), 7.24-8.16 (m, 12H, Ar-H), 8.84 (s, 2H, benzochromene-H4), 9.65 (s, 2H, 2NH), 10.49 (s, 2H, 2NH) |
25.51 (1C.), 27.33 (1C.), 28.69 (1C.), 34.62 (1C.), 119.28, 121.08, 122.43, 123.08, 126.13, 129.60, 132.87, 133.48, 145.23, 153.79 (Ar’C), 154.80, 158.25 (C=N), 167.33 (C=NH), 174.83 (C=O) |
|
17 |
3324, 3170, 3022, 2942, 2853, 1673, 1608 |
1.29 (brs, 8H), 1.56 (m, 4H), 2.39 (t, 4H), 7.12-8.34 (m, 12H, Ar-H), 8.97 (s, 2H, 2NH.), 10.26 (s, 2H, 2NH) |
26.12 (1C.), 27.68 (1C.), 29.08 (1C.), 34.15 (1C.), 118.35, 123.62, 125.53, 126.42, 127.74, 128.36, 130.25, 132.10, 144.61, 154.52 (Ar’C), 155.75, 157.32 (C=N), 162.49 (C=NH), 172.78 (C=O) |
Results and Discussion
The starting material, N1,N10-bis(5-(cyanomethyl)-1,3,4-thiadiazol-2-yl)decane-diamide 4 was obtained via one-pot sequential reaction of sebacoyl chloride 1 with ammonium thiocyanate and cyanoacetic acid hydrazide 2 upon refluxing in dioxane. It is assumed that 1 reacts initially with sebacoyl isothiocyanate to yield the bis-(thiosemicarbazide) derivative 3 as intermediate which experienced cyclization to yield the bis(1,3,4-thiadiazole) 4 (Scheme 1).
Repeating the reaction in acetone as a solvent, the bis(1,3,4-thiadiazole) derivative 5 was obtained. IR spectrum (cm-1) of 4 revealed 3205 (NH), 2927, 2851 (aliphatic-H), 2263 (C≡N), 1685 (C=O). Its 1H-NMR spectrum (DMSO-d6, δ ppm) revealed a broad singlet signal at 1.26 corresponding to four methylene groups, a multiplet at 1.57 corresponding to two methylene groups, triplet at 2.36 corresponding to two CH2CO, 3.74 corresponding to CH2CN, and singlet at 10.13 attributed to imino group. 13C-NMR spectrum of 4 exhibited signals at 18.73, 25.06, 28.93, 29.04, 34.10 and 46.22 for the methylene carbons, a signal at 116.94 due to the cyano carbons, two signals at 154.07, 167.32 related to the C=N carbons, a signal at 172.20 ascribed to carbonyl carbons. 1H-NMR spectrum of 5 displayed signals at 2.23 and 2.41 corresponding to 2CH3 groups with a singlet at 10.16 attributed to NH group in addition to protons of sebacoyl moiety.
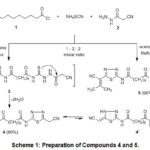 |
Scheme 1: Preparation of Compounds 4 and 5. |
Knoevenagel condensation of bis(1,3,4-thiadiazole) derivative 4 with aromatic aldehydes 6a-c afforded bis(arylidenes) 7a-c (Scheme 2). IR spectrum (cm-1) of 7a revealed 1687, 2215, 2853, 2939, 3017, 3190 attributed to C=O, C≡N, aliphatic, aromatic and imino functional groups. Its 1H-NMR spectrum exhibited signals at 8.45&10.12 due to benzylidene-H and imino protons together with aromatic protons at 7.04-7.35 in addition to the sebacoyl hydrogen’s.
Refluxing of compound 4 with arylidene-malononitriles 8a-c in ethanol/piperidine gave bis(1,3,4-thiadiazolo[3,2-a]pyridines) 9a-c in good yields. Compounds 9a-c were also achieved via refluxing of the arylidenes 7a-c with malononitrile under the same conditions. Compound 4 react with two equivalents of isatin in refluxing ethanol/piperidine and afforded bis(indoline) derivative 10 (Scheme 2).
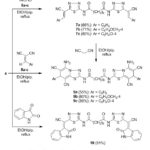 |
Scheme 2: Preparation of compounds 7a-c, 9a-c and 10 |
Ternary condensation of compound 4, elemental sulfur and phenyl isothiocyanate (in 1:2:2 molar ratio) in dioxane containing triethylamine afforded 11 (Scheme 3). The construction of the product took place in parallel to the reported Hantzsch reaction reported reaction29. The infrared spectrum (cm-1) of 11 showed significant absorption bands for NH2 and NH groups at 3350, 3240, 3161, vC=O at 1690, and vC=N at 1619. 1H-NMR spectrum displayed signals characteristic for imino proton at 10.10, aromatic protons as multiplet at 7.34-7.63, and singlet at 6.58 NH2 group disappeared with D2O, together with other signals which are regular with the suggested structure. Moreover, cyclocondensation of 4 with thioglycollic acid in acetic acid at reflux temperature gave bis(4-thiazolidinone) derivative 12 in good yield (Scheme 3). IR spectrum (cm-1) displayed the lack of a carbonitrile band and presence of absorption bands at 3209, 3051, 2923, 2851, 1732, 1681 corresponding to NH, aromatic, aliphatic, and carbonyl functional groups. 1H-NMR spectrum indicated the presence of two singlets of equal integrals at 3.84 and 4.13 which was corresponding for two different methylene groups with singlet at 10.15 assigned for 2 NH beside presence of sebacoyl hydrogen’s. The construction of compound 12 may be supposed to form via primary addition of the mercapto group in the reagent to the cyano group in compound 4 which undergo intramolecular cyclization by loss of two water molecules.
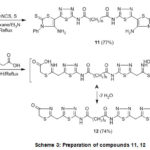 |
Scheme 3: Preparation of compounds 11, 12 |
Chromene derivatives were reported to have a widespread biological properties30. Thus, cyclocondensation of compound 4 with salicylaldehyde and/or 2-hydroxynaphthaldehyde in ethanol/ammonium acetate gave bis(chromene) 14 and bis(benzochromene) 16 (Scheme 4). Moreover, cyclocondensation of 4 with salicylaldehyde in acetic acid catalyzed with fused sodium acetate gave bis(coumarin) derivative 15. Compound 15 was also prepared via hydrolysis of 14 with HCl. IR spectrum (cm-1) of 14 displayed absorption bands at 3283, 3135, 1678 assignable to NH&C=O functions. Its 1H-NMR spectrum exhibited two signals at 9.16 and 10.35 due to 2 NH protons, singlet at 8.50 due to chromene-H beside aromatic protons at 7.40-8.13 beside the protons of sebacoyl moiety. 1H-NMR spectrum of 15 exhibited D2O-exchangeable signal at 10.20 two CONH protons, in addition to sebecoyl protons. Finally, cyclocondensation of compound 4 with 1-nitroso- 2-naphthol in refluxing ethanol catalyzed with ammonium acetate gave bis(naphtho[2,1-b][1,4]oxazine) derivative 17 (Scheme 4). IR (cm-1) of 17 showed bands at 3324, 3170 (NH). Its 1H-NMR spectrum exhibited a multiplet at 7.12–8.34, ascribed to aromatic protons, and two D2O-exchangeable singlet at 8.97, 10.26 for NH protons.
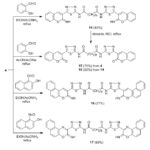 |
Scheme 4: Preparation of compounds 14-17 |
Conclusion
In summary, a facile and appropriate synthesis of some unique bis (thiadiazoles, 4-thiazolidinone, chromenes, 1,3,4-thiadiazolo[3,2-a]pyridines and naphtho[2,1-b]oxazines) containing sebacoyl spacer has been described. The chemical structure interpretations of the titled compounds were attained using elemental analyses, 1H&13C-NMR.
Acknowledgement
The author would like to express his deep gratitude to the Northern Border University, Kingdom of Saudi Arabia for providing financial support of this research (project 2017-1-F- 7038).
Conflicts of interest
There are no conflicts of interest.
References
- Saini, K.M.; Saunthwal, R.K.; Kumar, S. et al. Org Biomol. Chem., 2019; 17, 2657-2662.
- Manaka, A.; Ishii, T.; Takahashi, K.; Sato, M. Tetrahedron Lett., 2005; 46, 419-422.
- Piscitelli, F.; Ballatore C., Smith, A.B. Bioorg. Med. Chem. Lett., 2010; 20, 644-648.
- Diana, P.; Barraja P.; Lauria A. et al. Bioorg. Med. Chem., 2003; 11, 2371-2380.
- Sadchikova, E.V.; Bakulev, V.A.; Subbotina, J.O. et al. Tetrahedron, 2013; 69, 6987-6992.
- Varshney, K.; Gupta, A.K.; Rawat, A. et al. Chemical Biology & Drug Design, 2019; 94 (1): 1378-1389.
- Hemdan, M.M.; Fahmy, AF, El-Sayed, AA. J. Chem. Res., 2010; 34 (4), 219-221.
- Rezki, N.; Al-Yahyawi, A.M.; Bardaweel, S.K.; Al-Blewi, F.F.; Aouad, M.R.; Molecules, 2015, 20(9), 16048–16067.
- El-Sayed, W.A.; Metwally, M.A.; Nada, D.S. Abdel-Rahman A.A.H. J. Heterocycl. Chem., 2013, 50, 194–201.
- Bayrak, H.; Demirbas, A.; Demirbas, N.; Karaoglu, S.A. Eur. J. Med. Chem., 2010, 45(11), 4726–4732.
- Kadi, A.A.; Al-Abdullah, E.S.; Shehata, I.A.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A.; Eur. J. Med. Chem., 2010, 45(11), 5006–5011.
- El-Naggar, M.; Sallam, H.; Shaban, A. S. S.; Abdel-Wahab, S. S.; Amr, A. E.; Azab, M. E.; Nossier, E. S.; Al-Omar, M. A. Molecules, 2019, 24, 1066-1081.
- Altıntop, M. D.; Ciftci, H. I.; Radwan, M. O.; Sever, B.; Kaplancıklı, Z. A.; Ali, T. F. S.; Koga, R.; Fujita, M.; Otsuka, M.; Özdemir, A.; Molecules, 2018, 23, 59-75.
- Karakus, S.; Kocyigit-Kaymakcioglu, B.; Toklu, H.Z.; Aricioglu F, Rollas S. Arch. Pharm., 2009, 342, 48-53.
- Diaz, J.R.; Camí, G.E.; Liu-González, M.; Vega, D.R.; Vullo, D.; Juárez, A.; Pedregosa, J.C.; Supuran, C.T. J. Enzyme Inhib. Med. Chem., 2016, 31(6), 1102-1110.
- Jakovljević K, Joksović MD, Matić IZ, Petrović N, Stanojković T, Sladić D, Vujčić M, Janović B, Joksović L, Trifunović S, Marković V. Medchemcomm. 2018, 9(10), 1679-1697.
- El-Badry, Y, A.; Sallam M.S.; El-Hashash, M. A. Chem. Pharm. Bull. (Tokyo), 2018, 66(4), 427-433.
- Ramprasad, J.; Nayak, N.; Dalimba, U. Eur. J. Med. Chem., 2015, 106, 75-84
- Jubie, S.; Ramesh, P.N.; Dhanabal, P.; Kalirajan, R.; Muruganantham, N.; Antony, A.S. Eur. J. Med. Chem., 2012, 54, 931-935.
- El-Feky, S.A., Thabet, H.Kh. and Ubied, M.T. J. Fluor. Chem., 2014, 161, 87-94.
- Ammar, Y.A., Al-Sehemi, A.G., Ali, M.M., Mohamed, Y.A., Thabet, H.Kh., El-Gaby, M.S.A., Heterocycl. Lett., 2015, 5(2), 157-167.
- Ubied, M.T., Thabet, H.Kh., El-Feky, S.A. Heterocycl. Commun., 2016, 22(1), 43-47.
- Farrag, A.A., Ammar Y.A., El-Sehemi, A.G., Thabet, H.Kh., Hassan N. A.; Samy, A. Kh. J. Chem. Res., 2011, 35, 163-166.
- El-Feky, S. A., Abd El-Samii, Z. K., Osman, N. A., Lashine, J., Kamel M. A., Thabet, H. Kh. Bioorg. Chem., 2015, 58, 104-116.
- Elzahabi, H.S.A.; Salem, M.A. Thabet, H.Kh. Der Pharma Chem., 2011, 3, 48-58.
- Ammar, Y. A., Thabet, H. Kh., Ali, M. M., Mohamed Y. A., Ismail, M. A., Salem, M. A. Phosph. Sulfur Silicon, 2010, 185(4), 743-753.
- Hessein, S.A.; El-Sharief, M.A.M. Sh.; Abbas, S.Y.; Thabet, H.Kh.; Ammar, Y. A. Croat. Chem. Acta, 2016, 89(1), 91-100.
- Thabet, H.Kh.; Ubied, M.T.; El-Feky, S.A. J. Chem. Res., 2015, 39 (10), 567-573.
- Tormos, G.V.; Khodorkovsky, V.Yu.; Neilands, O.Ya., Belyakov, S.V. Tetrahedron, 1992, 48, 6863-6874.
- Ammar, Y.A.; El-Gaby, M.S.A.; Salem, M.A. Arabian Journal of Chemistry, 2014, 7, 615-622.

This work is licensed under a Creative Commons Attribution 4.0 International License.









