The use of Silica Supported Nickel-Copper Oxide Catalyst for Photodegradation of Methylene Blue
Anti Kolonial Prodjosantoso* , Farrasiya Indika Shelma, Kun Sri Budiasih and Maximus Pranjoto Utomo
, Farrasiya Indika Shelma, Kun Sri Budiasih and Maximus Pranjoto Utomo
Department of Chemistry, Yogyakarta State University, Yogyakarta, 55281, Indonesia.
Corresponding Author E-mail: prodjosantoso@uny.ac.id
DOI : http://dx.doi.org/10.13005/ojc/350423
Article Received on : 02-07-2019
Article Accepted on : 06-08-2019
Article Published : 19 Aug 2019
Photodegradation is a save and low cost methods to clean water bodies from some organic pollutants. The method has been developed in term of increasing the efficiency of the degradation capacity of photocatalyst. The photocatalyst working under visible light is the most desirable. The preparation of silica supported nickel-copper oxide [(Ni-Cu)Ox@SiO2] catalyst and the use of the catalyst in the photodegradation of methylene blue in the water are reported. The catalyst was prepared by impregnating the silica support into the mixture of nickel and copper salts, followed by calcination at 800○C for 4 hours. A series method of XRD, SEM-EDX, and UV-Vis Diffuse Reflectance has been used to characterize the catalyst. The catalyst adsorption test was undertaken in the dark, and the catalyst activity test for photodegradation of methylene blue was conducted under the sunlight. The XRD diffractogram of as prepared (Ni-Cu)Ox@SiO2 shows a weak-wide peak at 2θ = 21.8° indicating SiO2 tridymite, and has a crystallite size of 10.38 nm. The combination method of SEM and EDX confirms the formation of (Ni-Cu)Ox@SiO2. The (Ni-Cu)Ox@SiO2 catalyst has a relatively low bandgap energy and shows a good activity for photodegradation of blue methylene under sunlight. The adsorption of the methylene blue on the (Ni-Cu)Ox@SiO2 follows the Langmuir isotherm pattern.
KEYWORDS:Impregnation; (Ni-Cu)Ox@SiO2; SiO2
Download this article as:| Copy the following to cite this article: Prodjosantoso A. K, Shelma F. I, Budiasih K. S, Utomo M. P. The use of Silica Supported Nickel-Copper Oxide Catalyst for Photodegradation of Methylene Blue. Orient J Chem 2019;35(4). |
| Copy the following to cite this URL: Prodjosantoso A. K, Shelma F. I, Budiasih K. S, Utomo M. P. The use of Silica Supported Nickel-Copper Oxide Catalyst for Photodegradation of Methylene Blue. Orient J Chem 2019;35(4). Available from: https://bit.ly/33G0Yrg |
Introduction
Textile industry is one of the fastest growing industries in Indonesia.1 The rapid development of the textile industry poses a problem for the environment, especially the problem caused by textile dyeing liquid waste. The liquid waste contains toxic and dangerous compounds. The liquid waste in the waters can kill water organisms and block sunlight from penetrating the aquatic environment, thus disrupting the biological processes.2
Discharged dyestuffs from textile industry are generally in the form of organic compounds that are bio-nondegradable and difficult to be decomposed.3 Dyes often used in the textile industry are methylene blue, methyl orange, and Congo red. Methylene blue is a cationic heterocyclic aromatic compound commonly used as basic leather, cloth, and fabric coloring. The threshold limit value of methylene blue is 5-10 mg/L.
The conventional method for reducing the levels of methylene blue in water includes chlorination, ozonation, and adsorption using activated carbon.4,5 New methods which are easy to implement and relatively inexpensive need to be developed. One of these is photodegradation using a combination of semi-conductor photocatalyst and ultraviolet light.6,7 Harraz et al. (2019) was successful in engineering the photodegradation of methylene blue under UV and visible light using TiO2@SiO2 photocatalyst.8 The photodegradation will be more costly effective if undertaken under the sunlight.9
The use of photocatalyst has advantages because it can mineralize the total organic pollutants including the textile waste (azo compounds), the cost of their use is cheap and the process is relatively fast, non-toxic and has long-term use capability.9,10 The ability of the photocatalyst can be improved by applying materials such as TiO2, ZnO, Fe2O3, CdS, and ZnS which have semiconductor properties.11–13 However, the materials have a relatively expensive price. Therefore, semiconductor materials need to be supported by inexpensive compounds such as silica (SiO2).14,15
Nickel and copper oxides are semiconductor materials. The bandgap energy of NiO and CuO is >3 eV and 2-3 eV, respectively.16,17 To lower the bandgap of SiO2 which is about 9 eV the nickel and copper oxides may be supported on the surface of silica. Silica-supported nickel and copper oxides are expected to work as a photocatalyst of methylene blue under sunlight.
Materials and Methods
Materials including SiO2 (Merck, 99.3%), Ni(NO3)2.3H2O (Merck, 99.99%), Cu(NO3)2.3H2O (Merck, 99-104%), and Methylene Blue, are used without any prior treatment.
The (Ni-Cu)Ox@SiO2 catalyst was prepared by mixing a proportional amount of Ni(NO3)2.3H2O, Cu(NO3)2.3H2O and SiO2, and distilled water to a volume of 30 mL. The mixture was stirred using a magnetic stirrer and evaporated. Nearly dry mixture was added with ethanol, then methanol, stirred and filtered. The residue was air dried for 24 hours, and calcined at 800°C for 4 hours.
The XRD diffractogram of (Ni-Cu)Ox@SiO2 catalyst was obtained by using X-Ray. Diffraction Rigaku Multiflex, and Cu-Kα radiation in the range 2θ from 5°-80°. The UV-Vis spectrum of the samples were recorded as a function of wavelength using the UV-Vis 1700 Pharmaspec Spectrophotometer Specular Reflectance. The samples were put into cuvettes and the absorbances were measured in a wavelength range of 200-800 nm. The samples of (Ni-Cu)Ox@SiO2 were qualitatively analyzed using Scanning Electron Microscopy-Electron Dispersive X-Ray Analyzer JEOL JED-2300 working at 15 kV.
Dark adsorption test was undertaken by mixing 0.1 gram of the (Ni-Cu)Ox@SiO2 catalyst with 50 ml of 10 ppm methylene blue solution in a 100 mL Erlenmeyer. The mixture was stirred in a dark room. The absorption of the filtrate was measured at the time interval of 0, 5, 10, 15, 20, 25, 35, 65, 95, and 125 minutes, and at a wavelength of 663 nm.
Photocatalyst activity of the (Ni-Cu)Ox@SiO2 under sunlight was measured by mixing 0.1 gram of the catalyst with 50 ml of 10 ppm methylene blue solution in a 100 mL Erlenmeyer. The mixture was exposed under the sunlight, and the absorbance of the filtrate was observed after 0, 5, 10, 15, 20, 25, 35, 65, 95, and 125 minutes of the sunlight exposure, at a wavelength of 663 nm.
Results and Disscussion
The grayish white of (Ni-Cu)Ox@SiO2 catalysts were successfully synthesized. The XRD spectra indicate the presence of a broad-weak peak at 2θ = 21.8º (Figure 1.), which is a diffraction of SiO2 tridymite (JCPDS No. 39-1425). Crystal size of (Ni-Cu)Ox@SiO2 calculated using the Scherrer equation is 10.38 nm.18,19
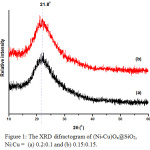 |
Figure 1: The XRD difractogram of (Ni-Cu)Ox@SiO2, Ni:Cu = (a) 0.2:0.1 and (b) 0.15:0.15. |
Unfortunately, the combination of SEM-EDX method is failed to prove the presence of both nickel and copper species on the surface of SiO2. The micrographs only indicate the silica species, that is SiO2 having particle size of between 0.1 – 15 µm (Figure 2). The EDX analysis suggests that both nickel and copper species are under limitation of EDX measurement (Figure 3).
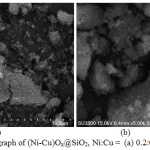 |
Figure 2: The SEM micrograph of (Ni-Cu)Ox@SiO2, Ni:Cu = (a) 0.2:0.1 and (b) 0.15:0.15. |
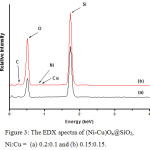 |
Figure 3: The EDX spectra of (Ni-Cu)Ox@SiO2, Ni:Cu = (a) 0.2:0.1 and (b) 0.15:0.15. |
UV-Vis Diffuse Reflectance method was applied to measure energy absorbance of samples at wavelength between 200-800 nm. It has been postulated that the UV spectrum ranges from 200-400 nm, while the visible spectrum ranges from 400-800 nm. All samples indicate peaks at UV and visible wavelenght (Table 1). These prove that the nickel and copper specieses are supported on the surface of SiO2, since SiO2 absorbs the energy at low wavelength.
Table 1: Absorbances of (Ni-Cu)Ox@SiO2 catalysts.
| (Ni-Cu)Ox@SiO2, Ni:Cu = | λ (nm) | |
| Visibel | UV | |
| 0.3:0.0 | 331 | 264 |
| 0.2:0.1 | 331 | 252 |
| 0.15:0.15 | 320 | – |
Bandgap energy affects the photocatalytic ability of (Ni-Cu)Ox@SiO2 catalyst. The reflectance data (REF) of the diffuse reflectance UV-Vis measurement is used in the calculation using the Kubelka-Munk equation to obtain the bandgap energy (Eg) of (Ni-Cu)Ox@SiO2.20 The calculation of bandgap energy by the Kubelka-Munk method include a graph of the relationship between eV and (F (R’∞xhυ))1/2 presented in Figure 4.
Figure 4 describes that bandgap energies of (Ni-Cu)Ox@SiO2, Ni:Cu = 0.3:0.0, 0.2:0.1 and 0.15:0.15 are between 3.40-2.58 eV, 4.62-2.31 eV, and 3.34-2.41 eV, respectively. Steiner, et al. (1992) found that NiO bandgap energy is ~4 eV,21 while the CuO has an energy bandgap between 1.85-2.4 eV and are effectively operated under UV and visible lights.22 This promises that the (Ni-Cu)Ox@SiO2 catalyst is also effectively used under sunlight.
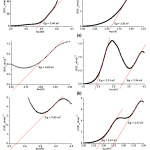 |
Figure 4: The calculation of (Ni-Cu)Ox@SiO2, Ni:Cu = (a) 0.3:0.0 (b) 0.2:0.1 and (c) 0.15:0.15, bandgap energies. |
The absorption study of (Ni-Cu)Ox@SiO2 on methylene blue is carried out in the dark room. This experiment facilitates the determination of the ability of the catalyst to absorb methylene blue without obstruction of light. The concentration of methylene blue before and after adsorption measured at any times is listed in Table 2.
Table 2: The concentration of methylene blue after adsorption by (Ni-Cu)Ox@SiO2.
| Time (minute) | The concentration of methylene blue (ppm) after adsorption by (Ni-Cu)Ox@SiO2, for Ni:Cu = | ||
| 0.3:0.0 | 0.2:0.2 | 0.15:0.15 | |
| 0 | 8.66727 | 8.66727 | 8.66727 |
| 5 | 5.49894 | 5.49894 | 5.49894 |
| 10 | 2.47603 | 2.37215 | 2.36176 |
| 15 | 1.88911 | 2.28905 | 2.13842 |
| 20 | 1.75926 | 1.87872 | 1.16714 |
| 25 | 1.74368 | 1.54630 | 1.11001 |
| 35 | 1.55669 | 1.52553 | 0.87628 |
| 65 | 1.43204 | 1.41126 | 0.55945 |
| 95 | 1.42684 | 1.37490 | 0.55438 |
| 125 | 1.01652 | 0.96977 | 0.54910 |
The (Ni-Cu)Ox@SiO2 catalyst absorption capacity was calculated by using the Langmuir and Freundlich equations.23 A plot of the concentration of methylene blue adsorbed in each 1 gram of catalyst (C/m) versus the final methylene blue concentration (C) is used to determine the Langmuir isotherm pattern, and a plot of the log amount of methylene blue adsorbed on every 1 gram of catalyst (log Xm/m) versus a log concentration of methylene blue after adsorption (log Ct) is used to determine the pattern of Freundlich isotherm. Line equations are obtained from the plots of Langmuir and Freundlich isotherms (Table 3). The values of R2 of Langmuir adsorption isotherm pattern are higher than the value of R2 of Freundich adsorption isotherm pattern, leading the conclusion that the adsorption of methylene blue by the (Ni-Cu)Ox@SiO2 follows the Langmuir isotherm pattern. This model assumes that there is only a surface layer (monolayer) and is homogeneous of (Ni-Cu)Ox on the surface of SiO2 which can block adsorption into deeper layers.
Table 3: Langmuir and Freundlich isotherm’s line equations.
| (Ni-Cu)Ox@SiO2, Ni:Cu= | Langmuir isotherm | Freundlich isotherm |
| 0.3:0.0 | y = 0.0743x – 0.0736 R² = 0.9757 |
y = -0.5398x + 1.6498 R² = 0.898 |
| 0.2:0.1 | y = 0.0734x – 0.0719 R² = 0.9695 |
y = -0.516x + 1.6422 R² = 0.8789 |
| 0.15:0.15 | y = 0.0665x – 0.0428 R² = 0.955 |
y = -0.369x + 1.5679 R² = 0.8247 |
The catalyst adsorption capacity can be calculated by using the line equation of the Langmuir isotherm pattern. The adsorption capacity of (Ni-Cu)Ox@SiO2 catalyst is listed in Table 4. The decreasing of NiOx and the increasing of CuOx is related linearly with the increasing of the adsorsobtion capacity of (Ni-Cu)Ox@SiO2 catalyst to methylene blue.
Tabel 4: The adsorption capacity of (Ni-Cu)Ox@SiO2 catalyst.
| (Ni-Cu)Ox@SiO2, Ni:Cu= | Adsorption capacity (mol/gram) |
| 0.3:0.0 | 13.5870 |
| 0.2:0.1 | 13.9082 |
| 0.15:0.15 | 23.3645 |
The concentration of methylene blue isolated in the dark for 24 hours is higher than for 2 hours, might be due to desorption process. The methylene blue in the solution was then exposed to sunlight at period of times. The concentration of methylene blue after exposed by sunlight at period of times is presented in Figure 5. The concentation of methylene blue is gradually decreased at the first 20 minutes. The concentration is then steady figuring that the photodegration is finished. The percentages of methylene blue photodegradated under sunlight using (Ni-Cu)Ox@SiO2, where Ni:Cu=0.3:0, 0.2:0.1 and 1.5:1.5 are 55.65%, 49.87%, and 52.11%, respectively.
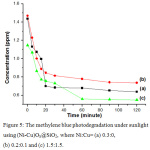 |
Figure 5: The methylene blue photodegradation under sunlight using (Ni-Cu)Ox@SiO2, where Ni:Cu= (a) 0.3:0, (b) 0.2:0.1 and (c) 1.5:1.5. |
The reaction order kinetics for photodegradation of methylene blue are presented in Table 5. The highest R2 values indicate the second reaction order. These is meant that the photodegradation reaction rate of methylene blue using the (Ni-Cu)Ox@SiO2 catalyst under sunlight follows the second-order reaction.
Table 5: Methylene blue photodegradation order kinetics.
| (Ni-Cu)Ox@SiO2, Ni:Cu= | Order | k | R2 |
| 0.3:0.0 | 0 | 0.0036 | 0.5293 |
| 1 | 0.0043 | 0.5565 | |
| 2 | 0.0053 | 0.5866 | |
| 0.2:0.1 | 0 | 0.0028 | 0.5148 |
| 1 | 0.0031 | 0.5812 | |
| 2 | 0.0036 | 0.6476 | |
| 0.15:0.15 | 0 | 0.0036 | 0.6903 |
| 1 | 0.005 | 0.7631 | |
| 2 | 0.0071 | 0.8169 |
The photocatalytic reaction takes place in a heterogeneous system and the reaction rate is influenced by the adsorbance of the reactants on the surface of the catalyst. The kinetic constant kobs is the reaction rate constant that does not take into account the role of adsorption, so that when the adsorption process becomes a part that affects the photodegradation reaction, it is necessary to determine the actual reaction rate constant (k) which has corrected the kobs with the adsorption constant (K) where kobs = kKL. The kinetics parameters of the kobs are determined by fitting the curve 1/Ct to the time due to the photodegradation reaction of methylene blue with (Ni-Cu)Ox@SiO2 following a second-order reaction. Then the reaction rate constant (k) is determined based on the kobs listed in Table 6. The (Ni-Cu)Ox@SiO2, Ni:Cu=0.15:0.15 shows the highest photodegradation reaction rate constant of methylene blue under sunlight, which is 0.10677•104 minute-1. This concludes that the (Ni-Cu)Ox@SiO2, Ni:Cu=0.15:0.15 is most effectively used as a photocatalyst under sunlight.
Table 6: Methylene blue photodegradation kinetics using Ni-Cu oxide@SiO2 catalyst.
| (Ni-Cu)Ox@SiO2, Ni:Cu= | KL | kobs (minute-1) | k (minute-1) |
| 0.3:0.0 | 0.0743 • 106 | 0.0053 | 0.07133 • 104 |
| 0.2:0.1 | 0.0734 • 106 | 0.0036 | 0.04905 • 104 |
| 0.15:0.15 | 0.0665 • 106 | 0.0071 | 0.10677 • 104 |
The photodegradation reaction rates of methylene blue are listed in Table 7. The difference in Ni and Cu concentrations supported onto silica does not have a significant effect on the photodegradation reaction rate equation. This proves that the (Ni-Cu)Ox@SiO2 catalyst is effective for photodegradation of methylene blue under sunlight.
Table 7: Methylene blue photodegradation rate.
| (Ni-Cu)Ox@SiO2, Ni:Cu= | Reaction rate (ppm/minute) |
| 0.3:0.0 | 0.07133 • 104×C2 |
| 0.2:0.1 | 0.04905 • 104×C2 |
| 0.15:0.15 | 0.10677 • 104×C2 |
Conclusion
The as prepared (Ni-Cu)Ox@SiO2 is representative for photodegradation of methylene blue. The nickel and copper oxides decrese the bandgap of silica making the catalyst suitable for photodegradation under visible and ultra violet lights. The catalyst adsorption follows the Langmuir isotherm pattern with adsorption capacity of (Ni-Cu)Ox@SiO2 increases along with the increasing of copper oxide. The photocatalytic activity of the catalyst in the degradation of methylene blue under the sunlight follows a second order reaction rate.
Acknowledgements
This research was supported and fully funded by the Yogyakarta State University of Indonesia.
Conflict of Interest
There is no conflict of interest.
References
- Paraschiv, D.; Tudor, C.; Petrariu, R., Sustainability , 2015, 7, 1280–1291.
- Ananthashankar, R.A.G., J. Chem. Eng. Process Technol., 2013, 05, -.
- Kant, R., Nat. Sci., 2012, 04, 22–26.
- Aljeboree, A. M.; Alshirifi, A. N.; Alkaim, A. F., Arab. J. Chem., 2017, 10, S3381–S3393.
- Konsowa, A. H.; Ossman, M. E.; Chen, Y.; Crittenden, J. C., J. Hazard. Mater., 2010, 176, 181–185.
- Zhou, X.T.; Ji, H.B.; Huang, X.J., Molecules, 2012, 17, 1149–1158 .
- Wan, J.; Sun, L.; Liu, E.; Fan, J.; Hu, X.; Pu, C.; Li, J.; Du, X., Mater. Lett. 2016, 169, 189-192.
- Harraz, F. A.; Mohamed, R. M.; Rashad, M. M.; Wang, Y. C.; Sigmund, W., Ceram. Int., 2014, 40, 375–384.
- Borges, M. E.; Sierra, M.; Cuevas, E.; García, R. D.; Esparza, P., Sol. Energy, 2016, 135, 527–535.
- Tian, C. Chem. Commun., 2012, 48, 2858.
- Soltani, N.; Saion, E.; Hussein, M.Z.; Erfani, M.; Abedini, A.; Bahmanrokh, G.; Navasery, M.; Vaziri, P., Int. J. Mol. Sci., 2012, 13, 12242-12258.
- Khaki, M. R. D.; Shafeeyan, M. S.; Raman, A. A. A., Journal of Environmental Management, 2017, 198, 78–94.
- Tada, H.; Jin, Q.; Nishijima, H.; Yamamoto, H.; Fujishima, M.; Okuoka, S.; Hattori, T.; Sumida, Y.; Kobayashi, H., Angew. Chemie Int. Ed., 2011, 50, 3501–3505.
- Maučec, D.; Maučec, D.; Šuligoj, A.; Ristić, A.; Dražić, G.; Pintar, A.; Tušar, N.T., Catal. Today, 2018, 310, 32–41.
- Chen, Y.; Wang, K.; Lou, L., J. Photochem. Photobiol. A Chem., 2004, 163, 281–287.
- Koshy, J.; Samuel, M.S.; Chandran, A.; George, K.C.; Predeep, P.; Thakur, M.; Varma, M. K. R., AIP Conference Proceedings , 2011, -, 576–578.
- Fujita, S.; Kawamori, H.; Honda, D.; Yoshida, H.; Arai, M., Appl. Catal. B Environ.,2016, 181, 818–824.
- Monshi, A.; Foroughi, M. R.; Monshi, M. R., World J. Nano Sci.. Eng, 2012, 02.
- Holzwarth, U.; Gibson, N., Nat. Nanotechnol., 2011, 6, 534–534 .
- Myrick, M.L.; Simcock, M.; Baranowski, M.; Brooke, H.; Morgan, S.; McCutcheon, J., Appl. Spectrosc. Rev., 2011, 46, 140–165.
- Hűfner, S.; Steiner, P.; Sander, I.; Reinert, F.; Schmitt, H., Zeitschrift fűr Phys. B Condens. Matter.; 1992, 86, 207–215.
- Abdel Rafea, M.; Roushdy, N., J. Phys. D. Appl. Phys., 2009, 42, 015413.
- LeVan, M. D.; Vermeulen, T., J. Phys. Chem., 1981, 85, 3247–3250.

This work is licensed under a Creative Commons Attribution 4.0 International License.









