Ferrocenyl Substituted Pyrazoles, Synthesis Via Novel Route, Spectral Investigations and Their Biological Studies
Manoj Kumar*1 , Shashi Sharma1, Hardeep Singh Tuli2 and Vinit Parkash1
, Shashi Sharma1, Hardeep Singh Tuli2 and Vinit Parkash1
1Department of Chemistry, M M University, Sadopur-Ambala, Haryana, India.
2Department of Biotechnology, M M D University, Mullana-Ambala, Haryana, India.
Corresponding Author E-mail: manojraju27@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350250
Article Received on : 06-02-2019
Article Accepted on : 06-04-2019
Article Published : 29 Apr 2019
Ferrocenyl substituted heterocyclic compounds have wide range of medicinal approach. The synthesis of ferrocenyl substituted pyrazole is the new concern in these compounds with enhanced biological activities. This work focus on synthesis of ferrocenyl substituted pyrazoles via novel route. The synthesis of 1-phenyl-3-ferrocenyl-pyrazole was investigated involving Friedel Crafts Acylation like reaction conditions. The reaction proceeded through three stages using addition cyclo-condensation of acetyl ferrocene with phenyl hydrazine followed by cyclization using cyclizing reagent iodine in presence of NaHCO3. Individual product separated out having excellent yield (83%). Ferrocenyl substituted pyrazoles were characterized by spectroscopic methods (1H NMR, IR, GC-MS) and their biological properties have been screened.
KEYWORDS:Addition Cyclo-Condensation; Acylation; Biological-Activities; Ferrocene; Pyrazole
Download this article as:| Copy the following to cite this article: Kumar M, Sharma S, Tuli H. S, Parkash V. Ferrocenyl Substituted Pyrazoles, Synthesis Via Novel Route, Spectral Investigations and Their Biological Studies. Orient J Chem 2019;35(2). |
| Copy the following to cite this URL: Kumar M, Sharma S, Tuli H. S, Parkash V. Ferrocenyl Substituted Pyrazoles, Synthesis Via Novel Route, Spectral Investigations and Their Biological Studies. Orient J Chem 2019;35(2). Available from: https://bit.ly/2IKzvxi |
Introduction
Ferrocene are interesting and important class of molecules because of their high thermal stability, variable oxidation states, aromatic character and iron source. These properties make it more favourable for drug designing. From literature glance various alkaloids, vitamins, antibiotics are heterocyclic in nature thus heterocylic compounds have many pharmaceutical advantages. Ferrocenyl moiety increases the biological activity of heterocyclic compound basically in inhibition of tumour cell growth.1-5
Ferrocenyl substituted pyrazole and similar series of substituted Ferrocenyl pyrazole synthesized from (2-formyl -1-chlorovinyl ) Ferrocene.6 in 2005 by Goremen et.al. In 2007, 5-alkyl-2ferrocenyl 6, 7-dihydropyrazolo[1,5-a]pyrazin-4[5H]-one complex and its derivatives having inhibitory effect towards A549 cancer cell growth was discovered[7]. New ferrocenyl derivatives of pyrazole analogues were prepared in 2014. One of them was (2-formyl-1-chlorovinyl)ferrocene prepared by using acetylferrocene. These compounds have anticancer and other biological activities.8
(R)-N,N-dimethyl-1-[(S)-2-{3-(1-phenyl)-1H-pyrazolyl}ferrocenyl]ethylamine was synthesized from 1-(R)-N,N-dimethylferrocenylethylamine in 1999 by Burkhardt et. al. This research was a new approach in the fieldof chelating pyrazole-containing ligands.9 Generally carbonyl compounds are raw material for the synthesis of many heterocyclic compounds but present study deals with acylation of ferrocene using little bit similar to Friedel Crafts Acylation. Novel approach reported in this study composed of two step synthesis for preparation of Ferrocenyl substituted pyrazole . Although many Friedel Craft acylation of ferrocene have been reported10-14 earlier but this route is quite unique. This study explore novel route for synthesis of ferrocenyl substituted pyrazole under optimizing conditions.
Materials and Method
All the chemicals used were of analytical grade procured from LOBA, MERK and OTTO. Solvents were distilled before use. Double distilled water is used. 1H NMR spectra was recorded on BRUKER ADVANCE II 400 NMR Spectrometer at 400MHz. IR spectra was made using Perkin Elmer – Spectrum RX-IFTIR instrument samples were prepared as KBr pellets. Mass spectra(m/z) was made by using Gas Chromatography Mass Spectrometry through SAIF LAB Chandigarh.
Synthesis
Synthesis of cyclopenta-2,4-dien-1-yl(2-(1-phenyl-1H-pyrazol-4-yl)cyclopenta-2,4-dien-1-yl)iron(III)
For the synthesis of 1-phenyl-3-ferrocenyl pyrazole Friedel Craft Acylation like technique was used. Although solubility of ferrocene created a problem because as such ferrocene was not soluble in acetic acid yet this situation was overcome by treatment of ferrocene with a mixture of concentrated HCl and acetic acid. A mixture of acetic acid (0.16mmol) and hydrochloric acid (0.13mmol) was treated with (0.005mole) ferrocene in presence of a pinch of Lewis acid (AlCl3 ) by refluxing with vigorous stirring at 100°C for two hour using magnetic stirrer with hot plate resulting in the synthesis of acetoferrocene.
Phenylhydrazine was added drop wise to acetoferrocene followed by refluxing with stirring using magnetic stirrer with hot plate at 60°C for one hour. Reaction was carried out in addition -Cyclo manner with removal of water molecule. 1-phenyl-3-ferrocenyl pyrazole was obtained as yellow solid by treatment the reaction mixture with iodine crystal (0.001mol) and 0.02mol) of sodium bicarbonate by refluxing at 100°C for three hours followed by quenching the reaction in ice.
IR (KBr, in cm-1): 416(Cp-Fe-Cp Str.), 750(C-H Str.), 1148(C-N Str.), 1661(C=N Str.). 1H nmr: (400 MHz, DMSO, in ppm) δ:6.69-6.79(m,5H,Cp), δ:1.93(s,1H,allylic-H), δ:7.27-7.31(d,1H,Cis-H), δ:1.99-2.00 (d,1H,allylic-H), δ:8.75(s,1H,N-H), δ:4.30(s,1H,vinyl H), δ:2.48(s,1H,CH-N), δ:7.00-7.16(m,5H,Ar-H). GC-MS (m/z): 328.02 found 328.17. Anal.Calc. for C19H1756FeN2: C, 69.54; H, 5.18; N, 8.54%. Found C, 69.09; H, 5.27; N, 8.19%.
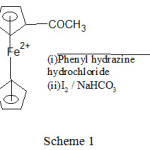 |
Scheme 1 |
Synthesis of cyclopenta-2,4-dien-1-yl(2-(1H-pyrazol-4-yl)cyclopenta-2,4-dien-1-yl)iron(III)
1 (1-hydride pyrazole)ferrocene was synthesized by addition cyclo–condensation manner. Acetoferrocene was treated with (0.1M) hydrazine drop wise with vigorous stirring at room temperature for half an hour and mixture left undisturbed so that reaction ceases. Then the reaction mixture was refluxed using magnetic stirrer with hot plate to 100°C for two hours.
Resultant product was obtained by treating reaction mixture with Iodine crystals (0.001 mol) and 0.02 mol) of sodium bicarbonate contained in a round bottom flask is refluxed using water bath to 100°C for 2.5 hour followed by reaction in ice and solid was separated out.
IR (KBr, in cm-1): 450,496(Cp-Fe-Cp Str.), 755(C-H Str.), 1158(C-N Str.), 1656(C=N Str.), 3260(N-H Str.), 3920(M-H asym. Str). 1HNMR (400 MHz, DMSO, in ppm): δ:6.62-6.69(m,5H,Cp), δ:1.83(s,1H,allylic-H), δ:7.17-7.21(d,1H,Cis-H), δ:8.77(s,1H,N-H), δ:4.33(s,1H,vinyl–H), δ:2.38(s,1H,CH-N), δ:7.01(s,1H). GC-MS (m/z): 252, found 252.59. Anal.Calc. for C13H1256FeN2: C, 61.95; H, 3.99; N, 11.11%. Found: C, 62.01; H, 3.90; N, 11.00 %.
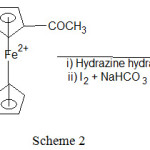 |
Scheme 2 |
Synthesis of bis(2-(1-phenyl-1H-pyrazol-4-yl)cyclopenta-2,4-dien-1-1yl)iron(III)
Bis(2-(1-phenyl-1H-pyrazol-4-yl)cyclopenta-2,4-dien-1-1yl)iron(III) was synthesized by diacetyl ferrocene treated with phenyl hydrazine hydrochloride. Diacetyl ferrocene mixed with phenyl hydrazine hydrochloride until the mixture became homogeneous at room temperature. Then the mixture was refluxed using magnetic stirrer with hot plate to 100°C for 3 hour.
1,1bis(1, 1-di-phenyl-3-ferrocenyl pyrazole was obtained by treating reaction mixture with Iodine crystals (0.001mol) and 0.02mol) of sodium bicarbonate by refluxing to 100°C for three hour using magnetic stirrer with hot plate. Then hot mixture was poured in ice a crude solid was separated out and recrystallized with ethyl alcohol. Then new product 1,1-bis(1, 1-di-phenyl-3-ferrocenyl pyrazole was carried out.
IR (KBr, in cm-1): 422(Cp-Fe –Cp Str.), 752(C-H Str.), 1133(C-N Str.), 1655(C=N Str.), 3259(N-H Str.), 3941(M-H asym. Str).1HNMR (400MHz, DMSO) δ:6.44-6.49(m,5H,Cp), δ:1.96(s,1H,allylic-H), δ:7.07-7.11(d,1H,Cis-H), δ:1.98-2.00(d,1H,allylic-H), δ:8.73(s,1H,N-H), δ:4.30(s,1H,vinyl–H), δ:2.48(s,1H,CH-N), δ:7.00-7.16(m,5H,Ar-H) GC-MS (m/z): 473, found 473.18. Anal. Calc. for C28H2456FeN4: C,71.06; N, 11.84; H, 5.07%. Found C, 71.79; N, 11.34; H, 5.27%.
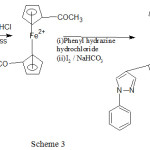 |
Scheme 3 |
Biological Study
Antimicrobial Assay
In vitro antimicrobial activity has been evaluated for synthesized ligand against pathogenic strains of bacteria (Staphylococcus aureus, Klebsiellapneumoniae) and fungi (Aspergillusniger, Trichophytonrubrum) using disc plate diffusion assay. The stock solutions of ligand (1000 ppm) was prepared in DMSO. The plates of culture medium using nutrient agar and potato dextrose agar were prepared for bacterial and fungal growth respectively under sterilized condition. The various concentrations of ligand (100, 150, 200 and 250 ppm) were loaded on 5 mm sterilized filter paper discs and placed on agar plates followed by incubation at 30°C for 24 h and 72 h to evaluate the effect of compound on bacterial and fungal growth respectively. Neomycin and Fluconazole were used as standard antimicrobial agents for bacterial and fungal study respectively.
DNA Photo-Cleavage Assay
DNA cleavage activity of the synthesized ligands were studied by agarose gel electrophoresis using supercoiled pUC19 plasmid DNA. The total volume of reaction mixture was 10 µl containing 0.5 µg of plasmid DNA in TE (Tris 10mM, EDTA 0.01mM, pH 8.0) buffer with various concentrations of synthesized ligands. The eppendorfs carrying reaction mixture were placed directly on the surface of a trans-illuminator (8000 mW/cm), at 360 nm for 30 min. After irradiation, samples were further incubated at 37°C for 1 h. Irradiated samples were mixed with 6X loading dye containing 0.25% bromophenol blue and 30% glycerol. The samples were then analyzed by electrophoresis on a 0.8% agarose horizontal slab gel in Tris-Acetate EDTA buffer (40 mMTris, 20 mM acetic acid, 1 mM EDTA, pH: 8.0) with comparison to untreated plasmid DNA as a control. Gel was stained with ethidium bromide (1 µg/ml) and photographed under UV light.
Result and Discussion
Spectral study of cyclopenta-2,4-dien-1-yl(2-(1-phenyl-1H-pyrazol-4-yl)cyclopenta-2,4- dien-1-yl)iron(III)
IR study: (cm-1) Eclipsed Ferrocene shows signal in region (400-500) for ten localised Fe-C bonds which is at 416 here. For aromatic C-H stretching out of plane bending is in between (900-690) which is at 750, C-N stretching is in region (1350-1000) which is at 1148, C=N stretching in region(1690-1640) which found in 1661 ,while N-H stretching is lies in between (3500-3100) meanwhile here is at 3264 , asymmetric M-H str. is at 3939.15-17 1H NMR study: (400MHz, DMSO, ppm) δ:6.69-6.79 (m,5H,Cp), δ:1.93 (s, 1H, allylic-H), δ:7.27-7.31 (d, 1H, Cis-H), δ:1.99-2.00 (d,1H,allylic-H), δ:8.75(s,1H,N-H), δ:4.30 (s,1H,vinyl H), δ:2.48 (s,1H,CH-N), δ :7.00-7.16 (m,5H,Ar-H). cyclopentadienyl ring give chemical shift in between 6- 8ppm in this value is 6.69 to 6.79 as a multiplet, allylic hydrogen have shift value ranging from 1.6-2.6ppm this peak is identified at 1.93 as a singlet, aromatic cis proton have shift value in region of 6-8ppm which is a doublet at 7.27 to 7.31, in the actual NMR spectra of acetylferrocene cyclopentadienyl ring has sharp singlet at 4.19 and singlet at 2.39 is for three equivalent protons of acetyl methyl group. Multiplets at 4.49 and 4.77ppm integrating for two types of protons in other cyclopentadienyl ring.18-19 GC-MS: Calc: C19H1756 FeN2: 328.02 found: 328.17.
Spectral study of cyclopenta-2,4-dien-1-yl(2-(1H-pyrazol-4-yl)cyclopenta-2,4-dien-1-yl) iron(III)
IR study: Eclipsed Ferrocene shows signal in region (400-500) for ten localised Fe-C bonds which is at 450 and 496. Aromatic C-H stretching out of plane bending is in between (900-690) which is at 755, C-N stretching is in region (1350-1000) which is at 1158, C=N stretching in region(1690-1640) which found in 1656, while N-H stretching is lies in between (3500-3100) meanwhile here is at 3260, asymmetric M-H str. is at 3920.20-21 1H NMR study: (400M Hz, DMSO, ppm) δ:6.62-6.69 (m,5H,Cp), δ:1.83 (s, 1H, allylic-H), δ:7.17-7.21 (d, 1H, Cis-H), δ:8.77 (s,1H,N-H), δ:4.33 (s,1H,vinyl–H), δ:2.38 (s,1H,CH-N), δ:7.01(s,1H). cyclopentadienyl ring give chemical shift in between 6-8ppm in this value is 6.62 to 6.69 as a multiplet, allylic hydrogen have shift value ranging from 1.6-2.6ppm this peak is identified at 1.83 as a singlet, aromatic cis proton have shift value in region of 6-8ppm which is a doublet at 7.17 to 7.21, N-H peak is identified at 8.77 meanwhile CH-N peak is at2.38.22-23 GC-MS: Calc: C13H1256 FeN2: 252.03 found 252.09.
Spectral study of bis(2-(1-phenyl-1H-pyrazol-4-yl)cyclopenta-2,4-dien-1-1yl)iron(III)
IR study: Eclipsed Ferrocene shows signal in region (400-500) for ten localized Fe-C bonds which is at 422. Aromatic C-H stretching out of plane bending is in between (900-690) which is at 752, C-N stretching is in region (1350-1000) which is at 1133, C=N stretching in region(1690-1640) which found in 1655 ,while N-H stretching is lies in between (3500-3100) meanwhile here is at 3259, asymmetric M-H str. is at3941.24-25 1H NMR study: (400MHz, DMSO, ppm) δ: 6.44-6.49 (m,5H,Cp), δ:1.96 (s,1H,allylic-H), δ:7.07-7.11(d,1H,Cis-H), δ:1.98-2.00 (d,1H,allylic-H), δ:8.73 (s,1H,N-H), δ:4.30 (s,1H,vinyl–H), δ:2.48 (s,1H,CH-N), δ:7.00-7.16 (m,5H,Ar-H) cyclopentadienyl ring give chemical shift in between 6-8ppm in this value is 6.44 to 6.49 as a multiplet, allylic hydrogen have shift value ranging from 1.6-2.6ppm this peak is identified at 1.96ppm as singlet, aromatic cis proton have shift value in region of 6-8ppm which is a doublet at 7.07 to 7.11.26-28 GC-MS : Calc:C28H2456 FeN4: 473.05 found 473.17.
Antimicrobial Activities
The antimicrobial effect of synthesized ferrocenyl substituted pyrazoles towards harmful human pathogenic microorganisms has been studied with comparison to Neomycin and Fluconazole. The compounds were found very potent against tested bacteria and fungi (fig.1). The antimicrobial action of ferrocenyl substituted pyrazoles was measured in term of zone of inhibition (Table 1 to 3) and MIC values were found to be in the range of 75-90 µg/ml. Previous evidences have supported that pyrazole based derivatives possesses broad spectrum of antimicrobial effects. Bekhit and Abdel-Aziem investigated the antimicrobial effect of novel pyrazole.29 derivatives against gram positive and gram negative bacterial as well as pathogenic fungi. Recently Mandal et al. and Munyaneza et al. have also reported the promising antimicrobial activities of metal complexes of pyrazole.30-31
 |
Figure 1: Antimicrobial Study of ferrocenyl substituted pyrazoles. |
Table 1: Antimicrobial effects of cyclopenta-2,4-dien-1-yl(2-(1-phenyl-1H-pyrazol-4-yl)cyclopenta-2,4-dien-1-yl)iron(III) against bacterial and fungal strains.
| Bacterial Strains(Zone of inhibition in mm) | Fungal Strains(Zone of inhibition in mm) | |||||||||||||||
| S. aureus | K. pneumoniaae | A. niger | Trichophytonrubrum | |||||||||||||
| Dosages | 100 ppm | 150 ppm | 200 ppm |
250 ppm | 100 ppm | 150 ppm | 200 ppm | 250 ppm | 100 ppm | 150 ppm | 200 ppm | 250 ppm | 100 ppm | 150 ppm | 200 ppm | 250 ppm |
| Zone of Inhibition | 11 mm ±0.5 | 15 mm±0.7 | 17 mm±0.6 | 18 mm±0.6 | 11 mm±0.5 | 13 mm±0.5 | 16 mm±0.5 | 18 mm±0.7 | 12 mm±0.5 | 15 mm±0.5 | 17 mm±0.5 | 20 mm±0.5 | 11 mm±0.7 | 13 mm±0.4 | 15 mm±0.5 | 17 mm±0.6 |
| Standards | 21 mm (Neomycin) | 23 mm (Neomycin) | (24 mm) Fluconazole | (9 mm) Fluconazole | ||||||||||||
Table 2: Antimicrobial effects of cyclopenta-2,4-dien-1-yl(2-(1H-pyrazol-4-yl)cyclopenta-2,4-dien-1-yl)iron(III) against bacterial and fungal strains.
| Bacterial Strains(Zone of inhibition in mm) | Fungal Strains(Zone of inhibition in mm) | |||||||||||||||
| S. aureus | K. pneumoniaae | A. niger | Trichophytonrubrum | |||||||||||||
| Dosages | 100 ppm | 150 ppm | 200 ppm | 250 ppm | 100 ppm | 150 ppm | 200 ppm | 250 ppm | 100 ppm | 150 ppm | 200 ppm | 250 ppm | 100 ppm | 150 ppm | 200 ppm | 250 ppm |
| Zone of Inhibition | 9 mm ±0.5 | 13 mm±0.7 | 15 mm±0.6 | 17 mm±0.6 | 10 mm±0.5 | 12 mm±0.5 | 15 mm±0.5 | 17 mm±0.7 | 11 mm±0.5 | 14 mm±0.5 | 18 mm±0.5 | 19 mm±0.5 | 10 mm±0.7 | 12 mm±0.4 | 14 mm±0.5 | 16 mm±0.6 |
| Standards | 21 mm (Neomycin) | 23 mm (Neomycin) | (24 mm) Fluconazole | (9 mm) Fluconazole | ||||||||||||
Table 3: Antimicrobial effects of bis(2-(1-phenyl-1H-pyrazol-4-yl)cyclopenta-2,4-dien-1-1yl)iron(III) against bacterial and fungal strains.
| Bacterial Strains(Zone of inhibition in mm) | Fungal Strains(Zone of inhibition in mm) | |||||||||||||||
| S. aureus | K. pneumoniaae | A. niger | Trichophytonrubrum | |||||||||||||
| Dosages | 100 ppm | 150 ppm | 200 ppm | 250 ppm | 100 ppm | 150 ppm | 200 ppm | 250 ppm | 100 ppm | 150 ppm | 200 ppm | 250 ppm | 100 ppm | 150 ppm | 200 ppm | 250 ppm |
| Zone of Inhibition | 12 mm ±0.5 | 17 mm±0.7 | 19 mm±0.6 | 20 mm±0.6 | 14 mm±0.5 | 16 mm±0.5 | 18 mm±0.5 | 21 mm±0.7 | 13 mm±0.5 | 16 mm±0.5 | 20 mm±0.5 | 22 mm±0.5 | 11 mm±0.7 | 16 mm±0.4 | 18 mm±0.5 | 19 mm±0.6 |
| Standards | 21 mm (Neomycin) | 23 mm (Neomycin) | (24 mm) Fluconazole | (9 mm) Fluconazole | ||||||||||||
DNA Photo-Cleavage Assay
The photo-induced DNA cleavage has been carried out using super coiled plasmid DNA by gel electrophoresis. The super coiled DNA (form I) migrate relatively fast with comparison to nicked DNA (form II). The conversion of form I to form II was observed with the treatment of synthesized ferrocenyl substituted pyrazoles in comparison to untreated plasmid DNA (Fig. 2) which indicates that ferrocenyl substituted pyrazoles have significant DNA-cleavage potential. Previously, Han et al. have reported the role of pyrazole based ligands as promising DNA cleaving action.32 via hydrolytic mechanisms. More recently, Feng et al. have investigated DNA binding capabilities of pyrazole derivatives and suggested their promising role in designing the chemotherapeutic drugs.33
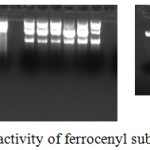 |
Figure 2: DNA Photo-cleavage activity of ferrocenyl substituted pyrazoles. |
Conclusion
Three ferrocenyl substituted pyrazoles have been synthesized via novel route and characterized. It is clear from the evidences that such bonding is possible. The results of the experimental study of ferrocenyl substituted pyrazoles give new approach towards new structural motif and lights the probability that these compounds can be explored as potential biological agents which leads to further new research.
Acknowledgements
The authors are thankful to MM University Trust, Ambala for the financial support during the course of this study.
Conflict of Interest
There is no conflict of interest.
References
- Bioani, M.; Gonzalez, M. Med. Chem. 2005, 5, 409-418.
- Lee, K.Y.; Kim , J.M.; Kim, J.N. Tetrahedron Lett . 2003, 44, 6737-6742.
- Jain, K.S.; Schitre, T.S.; Miniyar, P.B.; Kathiravan, M.K.; Bendre, V.S.; Veer, V.S.; Shahane, S.R.; Shishoo, C.J. Curr. Sci. 2006, 90, 793-799.
- Jin, Z. Nat. Prod. Rep. 2006, 23, 464-496.
- Melo, P.E.; Teresa, M.V.D. Curr. Org. Chem. 2005, 9, 925-958.
- Zora, M.; Gormen, M. Jour. Organ. Chem. 2007, 692,5026-5032
- Xia, Y.; Dong, Z.W.; Zhao, B.X.; Ge ,X.; Meng, N.; Shin, D.S. Bioorg. Med. Chem. 2007, 15, 6893–6899.
- Mandal, S.; Sadhukhan, R.; Ghosh, U.;Mandal,S.; Saha, M.; Ray J. Jour. Corrd. Chem. 2016, 69, 1618-1634.
- Burkhardt, U.; Drommi, D. Inorganic chimica Acta.1999, 296, 183-194.
- Bishop, B.C.; Brands, K.M.J.; Gibb, A.A.; Kennedy, D.J. Synthesis, 2004, 1, 43-52
- Szarka, Z.; Skoda-Foldes, R.; Balogh, J.; Kollar, L. Tetrahedron Lett. 2001, 42, 539-768.
- Szarka, Z.; Skoda-Foldes, R.; Kuik, A.; Berente, Z.; Kollar, L. Synthesis. 2003, 4, 545-550.
- Kuik, A.; Skoda- Foldes, R.; Balogh,J.; Kollar,L. J. Organomet. Chem. 2005, 690, 3237-3242.
- Balogh, J.; Zsoldos-Mady, V.; Frigyes, D.; Benyei, A.C.; Skoda Foldes, R.; Sohar, P. J. Organomet. Chem. 2007, 692, 1614-1618.
- Plazuk, D.; Zakrzewski, J. J. Organomet. Chem. 2009, 694, 1802-18069.
- Werner Angew, H. Chem. Int. Ed. 2012, 51, 6052-6058.
- Tirkey,V.; Mishra, S.; Dash, H.R.; Das,S.; Nayak ,B.P.; Mobin,S.N.; Chatterjee,S. J. Organomet. Chem. 2013, 732, 122-129.
- Maguene, G.M.; Jakhlal, J.; Ladyman, M.; Vallin, A.; Ralambomanana, D.A.; Bousquet, T.; Maugein, J.; Lebibi, J.; Pelinski, L. Eur. J. Med. Chem. 2011 , 46, 31-38.
- Astruc, D. Tetrahedron 1983, 39, 4027-4095.
- Sahu, K. S.; Banerjee, M.; Samantray, A.; Behera, C.; Azam, A.M. Tropical J. Pharma Res. 2008, 2, 961-968 .
- Holla, S.B.; Mahalinga, M.; Karthikeyau, S.M.; Akberali, M.P.; Shetty, S.N. Bioorg. Med. Chem. 2006, 14, 2040-2047.
- Katritzky, A.R.; Vakulenko, A.V.; Gedu, R.A.; Rogers, J.W. ARKIVOC i, 2007,60,8555-8560.
- Zhao, Li.J.; Zhao, Y.F.; Yuan, X.L.; Gong, P. Arech. Pharm. Chem. Life Sci. 2006, 339, 593-597.
- Yan, Z.R.; Liu, Y.X.; Xu F.W.; Pannecouque, C.; Trrouw, M.W.; Clereg, E.De. Arech. Pharm. Res. 2006, 29, 957-962.
- Perez-Rebolledo, A.; Ayala, J.D.; De Lima, G. M.; Marehini, N.; Bo-Mbieri, G.; Zani, C. L.; Souza-Fagundes E.M. Eur. J. Med. Chem. 2005, 40, 467-472.
- Tan, W.; Yu, Z.; He, W.; Wang, L.; Sun, J.; Chen, J. Organometallics . 2008 , 27, 4833-4836.
- Kumar, M.; Verma. G.R. Orient. J. Chem. 2010, 26, 517-525.
- Kumar, M.; Jaipal. Orient. J. Chem. 2007, 23, 705-712.
- Bekhit, A. A.; Abdel-Aziem, T. Bioorg. Med. Chem. 2004, 12, 1935–1945
- Mandal, S.; Mondal, M.; Biswas, J.K.; Cordes, D.B.; Slawin, A.M.Z.; Butcher, R.J.; Saha, M.; Saha, N.C. J. Mol. Struct. 2018, 1152, 189–198.
- Munyaneza, A.; Kumar, G. Morobe I.C. 2018, 3, 1-8.
- Han, C.; Guo, Y.C.; Wang, D.D.; Dai, X.J.; Wu, F.J.; Liu, H.F.; Dai, G.F.; Tao, J.C. Chin. Chem. Lett. 2015, 26, 534–538.
- Jiajia, F.; Hui ,Q.; Xiaoyang, Sun.; Siran, Feng.; Zhenming, Liu.; Yali, Song.; Xiaoqiang, Qiao. Chem. Pharm. Bull. 2018, 66, 1065-1071.

This work is licensed under a Creative Commons Attribution 4.0 International License.









