Characterization of Palladium Chelates and Their Interactions with Z-N'-(Benzo(d)Thiazol-2-yl)-N,N-Dimethylformimidamide using the Spectrophotometric and Computational Methods
Amr Lotfy Saber*1,2 , Wael Abd-Allah Zordok1,3, Ahmed Alharbi2 and Abdu Subaihi3
, Wael Abd-Allah Zordok1,3, Ahmed Alharbi2 and Abdu Subaihi3
1Department of Chemistry, Faculty of Science, Zagazig University, Zagazig, Egypt.
2Department of Chemistry, Faculty of Applied Science, Umm Al Qura University, Makkah, Kingdom of Saudi Arabia.
3Department of Chemistry, University College of Quanfudha, Umm Al -Qura University, Kingdom of Saudi Arabia.
Corresponding Author E-mail: alshefny@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/350217
Article Received on : 10-12-2018
Article Accepted on : 24-03-2019
Article Published : 27 Apr 2019
A rapid, selective and sensitive method for the quantification of Pd (II) using spectrophotometric technique associated with Z-N'-(benzo(d) thiazol-2-yl)-N,N-dimethylformimidamide BTDF as new chelating agent is described. Yellow colored complex of Pd (II) with BTDF is formed in Britton-Robinson universal buffer of pH 9 and extracted with chloroform. The formed complex clearly illustrate an absorption at ∼384 nm and follw Beer’s law in the range of concentration between 0.5–18.0 µg ml−1 of Pd (II) with absorbance of 3.62 × 104 L mol-1 cm-1 and limit of detection(LOD) of Pd (II) 0.07 µg ml−1. The effects of different experimental parameters have been established by study the optimum conditions for the extraction and quantification of palladium ion. Density functional theory (DFT) havealso been employed to computethe influence of the cation on theoretical parameters of Pd2+ complexes. The effect of donating centers was investigated theoretically which prove that Pd2+ favor coordinated with two molecules of Z-N'-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide through two nitrogen atoms.The performance of the examined method was estimated to detect the impact of current method over the presented methods in the literature without interference effect of cations and anions. The examined method has successfully demonstrated the quantification of Pd(II) in natural and spiked water samples.
KEYWORDS:Dimethylformimidamide; DFT; Palladium Ion; Spectrophotometry
Download this article as:| Copy the following to cite this article: Saber A. L, Zordok W. A, Alharbi A, Subaihi A. Characterization of Palladium Chelates and Their Interactions with Z-N'-(Benzo(d)Thiazol-2-yl)-N,N-Dimethylformimidamide using the Spectrophotometric and Computational Methods. Orient J Chem 2019;35(2). |
| Copy the following to cite this URL: Saber A. L, Zordok W. A, Alharbi A, Subaihi A. Characterization of Palladium Chelates and Their Interactions with Z-N'-(Benzo(d)Thiazol-2-yl)-N,N-Dimethylformimidamide using the Spectrophotometric and Computational Methods. Orient J Chem 2019;35(2). Available from: https://bit.ly/2VujEsG |
Introduction
Recently, the use of nitrogen containing heterocyclic derivativesin biological processes has attracted a lot of considerable attention, due to itsnatural abundance, chemistry coordinating ability for a trace metal ion and promising application in industry.1 The synthesis of platinum pyrimidine has become aninteresting complexes that can be isolated from the reaction of cisplatin with pyrimidines which have illustrated its effective for antitumor activity.2-7 The ligand display a great versatility when interact with metal ion and strong coordinating unit, due to its influence on the electronic and steric properties of the central metal, interesting metal-metal interactions in some cases of compounds.8 Depending on the conditions, reported study has indicated that pyrimidine ring is preferred binding to Pd(II) either in monodentate mode through one nitrogen atom commonly in a cis-configuration, or in bidentate mode through the two nitrogen atoms.9
Several studies have reported unprecedented compounds displayed new bonding modes for the pyrazolate ligands Pd(II) complexes.10-14 Only few studies in the literature have been demonstrated the utilize of pyrazolyl derivative complexes for assembly or self-assembly in extended arrays.15 Some derivatives of five-membered heterocycles have subjectedto considerable attention in the literature,16 in particular, studies on the synthesis of trifluoromethylsubstituted isoxazoles and pyrazoles.17-19 In this work, the interactions of Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide ligand with Pd(II) were investigated experimentally for the first time using a simple, easy to operate, reliable, highly sensitive and accurate extractive spectrophotometric method for the detection and quantification of Pd(II) in spiking different water samplesand these interactions were approvedusing quantum chemical calculations by calculating the HOMO/LUMO energy values and bond angles, bond lengths, charge density and dihedral angles of the complexes.
Materials and Methods
Apparatus
The spectra measurements for all samples were measured using a thermostatted Shimadzu UV-VIS-NIR-3600 double-beam spectrophotometer with wavelength range 150–1100 nm, spectral bandwidth 2.0 nm, with 10 mm matched quartz cells. The pH of solutions was tested using JENWAY Research Model 3510 pH Meter.
Reagents and Solutions
All of the chemicals utilized were of the highest purity analytical reagent grade. Double distilled water was utilized to prepare all the solutions.
BTDF was synthesized based on the method described previously.20 Standard stock solution (0.01 mol l-1) of Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide (BTDF) was prepared by dissolving 0.205 g of BTDF with methanol in a 100 ml calibrated flask. All solutions were freshly prepared at lower concentrations by suitable dilution with methanol.
Astock solution (0.01 mol l-1) of palladium(II) chloride was prepared by dissolving 0.1773 g of anhydrous PdCl2 in 100 ml of water acidified with few drops of concentrated HCl then the obtained solution was standardized by EDTA method (Merck, 1982). Working solutions were prepared by suitable dilution of the stock solution with distilled water.
The standard methods21 were used to prepare different series of Britton-Robinson(B-R) universal buffer solutions.
Recommended Procedure
An aliquot of sample containing 0.5–20 µg ml−1 of PdCl2 solution in desired acidity was transferred into a series of 50 ml separating funnels, 2.0 ml of (1 x 10-4 mol l-1) BTDF was added and then the total volume of the aqueous phase was adjusted to 10 ml using buffer solution pH 9 to give turbid yellowish solution of palladium complex then 10 ml of chloroform were added to each funnel and shaken process for the contents were done well for 5.0 min. The two layers were allowed to separate and the organic phase was dried over anhydrous sodium sulphate. The absorbance spectra of the solution was measured against BTDF/CHCl3 solution.
Quantification of Palladium Ion in Spiked and Natural Water Samples
Quantification of palladium (II) was performed in environmental samples that were previously checked negative for palladium (II). Then palladium (II)was spiked to tap water, lake and river samples at a concentration that obeyed the limit of Beer’s law for the analysis. To remove suspended particulate substances from natural water samples spiked with palladium (II) a 0.45 µm pore size membrane filter was used, after filtration the extraction efficiency for the recovery of palladium (II) was studied.The spectrophotometric measurements of final solution were carried out following the procedure mentioned above.
Results and Discussion
Palladium ion forms metal complex in acidic medium (pH 9) with Schiff base such as BTD Fand this complex are extracted quantitatively into chloroform. The spectra of absorption of the yellow metal complex solution were detected in the range 300-550 nm against the blank solution (Figure 2.). The palladium complex showsthe maximum absorbance at λmax as provided in (Table 1).
Table 1: Effect of order of reactants addition.
| S No. | Order of additiona | Absorbance for 1 ml of Pd(II)(1.0 x 10-4M) |
| 1 | A + B + C | 0.42 |
| 2 | A + C + B | 0.4 |
| 3 | B + A + C | 0.4 |
| 4 | B + C + A | 0.41 |
| 5 | C + A + B | 0.42 |
| 6 | C + B + A | 0.42 |
aA = BTDF, B = palladium(II), C = Buffer solution
Spectral Characteristics
In the current method a yellow colored of the extracted solution was formed with minimal interference,it was necessary to carry out the optimization to achieve the optimum wavelength for palladium(II) quantification. This wavelength should be specific monitoring and specific for Pd-BTDF quantification. The wavelength of maximum absorbance was obtained by measuring the yellow colored solution within the range 300-550 nm with various concentrations of palladium(III).
The optimum wavelength for achieving the best results was generated to be 384 nm and the reagent blanks had negligible absorption at the same wavelength. The absorption spectra of the metal complex is presented in Figure 1.
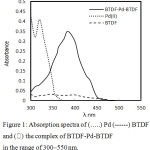 |
Figure 1: Absorption spectra of (…..) Pd (——) BTDF and (⸻) the complex of BTDF-Pd-BTDF in the range of 300–550 nm. |
Optimum Reagents Concentrations
The BTDF effect was examined by using a constant palladium (II) concentration in 10 ml standard flask. It was observed that (1 x 10-4 mol l-1) BTDF in the range of 0.5-3.0 ml was adequate to obtain the yellow color. Hence, 2.0 ml of (1 x 10-4 mol l-1) BTDF was preferred to achieve the maximum color intensity and utilized for further studies. Excellent data was generated when distilled water used for dilution and chloroform for extraction of the color product show more better than the other solvents.
The sensitivity of the proposed method increased by increasing BTDF volume up to 2.0 ml of (1 x 10-4 mol l-1) and decreased at higher volume due to this fact that the free BTDF competes with the complexes in extraction rich phase.
Effect of pH
The effect of pH on the formation of palladium (II)-BTDF complex and its absorbance was investigated by mixing 1.0 ml (1 x 10-4M) palladium (II), 2.0 ml (1 x 10-4M) BTDF and 5.0 ml pH 2 – 12 of universal buffer. The mixture was diluted to volume mark in a 10-ml standard flask with dist. water then the absorbance of the extracted yellow color complex was measured.
By comparing different types of buffer solution, the universal bufferillustrated the highest selectivity, sensitivity, stability and linearityof yellow colored product while the blank remained colorless in chloroform presence.The absorbance was maximum at pH 9.0 which was selected as optimal (Fig. 2).
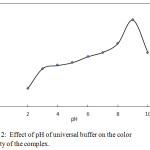 |
Figure 2: Effect of pH of universal buffer on the color intensity of the complex. |
Choice of Organic Solvent
A different organic solvents such as chloroform, dichloromethane, carbon tetrachloride, toluene, and benzene were tested for extraction of Pd-BTDF complex using applicable extraction procedure. The best appropriate solvent for the extraction of yellow colored complexes was chloroform that yielding maximum absorbance intensity.
Order of Reactants Addition
The influence of order of reactants addition was investigated by using varies orders of the reactants in optimized amounts. The data obtained has demonstrated that, the order of addition of reactants unaffected on the absorbance values as provided in Table 2, but for maintaining the similarity of the order of addition of reactants, series No.1 of Table 1 was followed throughout course of the quantification of palladium (II).
Table 2: Optical parameters for the determination of palladium(II).
| Parameter | Characteristic |
| pH | 9 |
| chloroform | |
| λmax | 384 |
| Molar ratio (Pd-BTDF) | 1 : 2 |
| Beer,s law limits (µg ml-1) | 0.5–18.0 |
| Molar absorptivity (l mol-1cm-1) | 3.62 × 104 |
| Sandell,s sensitivity (ng cm-2) | 17.2 |
| Regression equation* | |
| Intercept | 0.07 |
| Slope | 0.058 |
| Correlation coefficient (r) | 0.9994 |
| Range of error % | -0.48 : 0.61 |
| pK | 6.13 |
| Detection limit LOD(µg ml-1) | 0.053 |
| Quantification limit LOQ(µg ml-1) | 0.16 |
* A = a + bC, where C is the concentration in mg ml-1
Effect of Time and Temperature on Color Stability
In light of the optimized conditions, in spite of the color developed instantaneously, five minutes were adequate to get the constant and maximum absorbance, the colored complex was stable for one day. The measurements of absorbance changed by not more than 5% over a period of 30 hours and the color formation was independent of temperature in the range of 10-45°C. Hence, five minutes reaction at ambient temperature was adequate for the analysis.
Composition of Complex
Stoichiometric ratio of BTDF to PdCl2 in the complex wasobserved by the Job,s method of continuous variation using variable BTDF and palladium ion concentrations. The results showed that the stoichiometric ratio was 1:2 (Pd-BTDF) at the optimum conditions
Analytical Application
The determination of palladiumwas occurred using the proposed method by spiked water samples with known quantity of palladium (II) andstudy the recovery. The datagenerated by the current method was definite by measurements of palladiun contents using the reported methods.22 Student,s t-test and the variance ratio F-test at 95% confidence level were calculatedand showed that the calculationsdid not override those theoreticalvalues. Table 3 showed that there is no significant difference between those measured by proposed method and thespiked quantities and from the previous methods,referring that the current method is as accurateand precise as the previous methods. The comparison between the proposed method and some different reported methods hasshown that, the proposed method israpid, simple,and highly sensitive than thesemethods in survey provided in Table 4.
Table 3: Determination of palladium (II) in different water samples.
| Samples | Palladium added | Palladium found(μg ml-1) | t-testb F-testc | |
| μg ml-1 | Proposed method | Reported method | ||
| Founda Recovery | FoundaRecovery | |||
| Drinking water | 0.5 | 0.49 ± 1.20 98.0 | 0.503 ±1.45 100.6 | 0.94 1.83 |
| (Al Qassim-KSA*) | 2 | 1.98 ± 0.48 99.0 | 2.007 ± 0.65 100.3 | 0.96 1.07 |
| 4 | 4.02 ± 0.55 100.5 | 3.994 ± 0.29 99.8 | 0.44 1.57 | |
| Tab water | 0.5 | 0.498 ± 1.26 99.6 | 0.504 ± 1.66 100.8 | 1.28 1.85 |
| (Al Azizea-Makkah-KSA*) | 2 | 2.006 ± 0.57 100.3 | 2.004 ± 0.44 100.2 | 0.80 2.13 |
| 4 | 3.986 ± 0.54 99.7 | 3.997 ± 0.52 99.9 | 1.18 1.79 | |
| Tab water | 0.5 | 0.492 ± 1.36 98.4 | 0.504 ± 1.87 100.8 | 1.281.88 |
| (Alabdia-Makkah-KSA*) | 2 | 1.964 ± 0.44 98.2 | 1.994 ± 0.47 99.7 | 0.42 1.86 |
| 4 | 3.992 ± 0.33 99.8 | 3.991 ± 0.58 99.7 | 0.761.58 |
aMean ± Relative Standard Deviation (n = 5); b,cValues in parentheses are the theoretical values for t- and F- values at 95% confidencelimits and five degrees of freedom; * Kingdom of Saudi Arabia.
Table 4: Comparison of recommended method with reported methods.
| Reagent | λmax (nm) | Molar absorptivity, l mol-1 cm-1 | Conditions | Ref. |
| -Pyridoxal-4-phenyl-3-thiosemicarbazone. | 460 | 2.2×104 | Benzene, pH 3.0 | 53 |
| -Benzyloxybenzaldehyd ethiosemicarbazone. | 365 | 0.4×104 | Cyclohexanol, pH 5 | 54 |
| -N-ethyl-3-carbazolecarbax aledehyde-thiosemicarbazone. | 410 | 1.6×1104 | n-butanol, pH 4.0 | 55 |
| -Benzoin oxime. -2-(2-Quinolylazo)-5- | 330 | 4.0×103 | Hexone, 1 M HNO3 | 56 |
| dimethylaminoaniline | 600 | 13.5×104 | In 0.5–2.5M HCl | 57 |
| -N-Dodecyl-N0-(sodium-p-aminobenzenesulfonate)- thiourea | 296 | 7.41×104 | In CTMAB andNaAc–HAc buffer | 58 |
| BTDF | 384 | 3.62 ×104 | Chloroform, pH 9.0 | Present work |
Computational Details
Density functional theory (DFT) was utilized to study of the cation effect on theoretical parameters of the Pd2+ complexes of Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide and detect the exact structure of the complex results from coordination with metal ion. The Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide compound has posses four donating centers, three nitrogen atoms and one sulpher atom. The divalent metal ion Pd2+ may be chelated with two nitrogen atoms or chelated through the one nitrogen atom and one sulpher atom, so in this part we study the two complexes results from the two modes of chelation and show which is more stable than other. The pronounced effect of donating centers was examined theoretically and the excited state for entire compounds was investigated to establish the different types of electronic transitions which can be occurred on these compounds with different structure. The Frontier molecular orbitals were studied for its important role in the optical and electriccharacteristics, as well as in chemical reactions and UV-Vis. Spectra. Profiles of the geometry and optimal setof these complexes were studied using the GAUSSIAN 98W package of programs23 at Cep-31G level of theory.24
Computational Method
The geometric and energies parameters were studied at the B3LYP/Cep-31G level of theory by DFT, appling the GAUSSIAN 98W package of the programs, on geometries that were examined at Cep-31G basis set. The atomic charges were studied by the natural atomic orbital populations. The B3LYP is important for the hybrid functional,25 which shows a linear combination by Becke of the gradient functionals reported,26 Yang, Lee and Parr,27 together with local exchange function by Hartree-Fock.28
Model of Compounds and Structural Parameters
Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide
The chelating effect of this compound is mainly detected by its structure, the Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide has many characteristic structural features. The molecule is completely planer, also it is considered not highly sterically-hindered, there are two aromatic rings. The bond length C1-N3 is 1.369 Å, N2-C6 is 1.374 Å , also the C6-N7 is 1.317 Å there is a double bond characters C and N atoms,29 whereas C1-N2 is double bond 1.316Å.30 Analysis of investigated bond lengths in different sulfa molecules was given elsewhere.31 All distances and angles between the atoms of the sulfa compound system are given in Table (1). The S8-C10 and C6-S8 bond lengths are 1.788 Å and 1.796 Å32 respectively , the N3-C4 and N3-C5 bond lengths are 1.423 Å,33 also the N7-C9 bond length is 1.401 Å.33
The molecule is a completely planer and not highly sterically-hindered as seen in fig.(3), the Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide compound occupied one plan and all fragments are laying in the same plane, the dihedral angle C4N3C1N2, 179.99°C and C5N3C1N2, -0.00°C, it is means that the two terminal methyl groups are in the plane of central aromatic ring. The values of calculated dihedral angles: N3C1N2C6, 179.90°C, C1N2C6N7 and C1N2C6S8 are -179.90°C and 0.00°C respectively, so the aromatic ring lying in the plane of the molecule. Fig. (3), shows the optimized geometrical structure of Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide compound, the dihedral angles N2C6N7C9 is 179.90˚C and N2C6S8C10 is -179.90°C which confirms that the N2 and C1 lying in the same plane occupied by the two terminal methyl groups and lying in the same plane of the central aromatic ring.
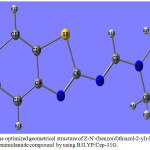 |
Figure 3: The optimized geometrical structure of Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide compound by using B3LYP/Cep-31G. |
The optimized geometrical parameters, bond distances, bond angles and dihedral angles of Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide as obtained from B3LYP/Cep-31G calculations are listed in Table (5). These data are drowning to give the optimized geometry of molecule as shown in Fig. (3). The bond angles, C4N3C5 is 121.22°C, C1N3C5 is 120.08˚, C1N3C4 is 118.69°C and N2C1N3 is 119.79°C and C1N2C6 is 120.34°C reflects on sp2 hybridization of the N3 atom. The values of bond distances are compared nicely with that obtained from X-ray data.29 Comparisons of the performance of varies DFT procedures allow outlining the principle trends of these theoretical approaches which are necessary to better understand reaction mechanisms of Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide compound and the properties. However, till now, no attempt has been made to analyze the application of different DFT steps and various basis sets for accurate calculations of structure of Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide.33
Table 5: Equilibrium geometric parameters bond lengths (Å), bond angles (˚), dihedral angles (˚) and charge density of Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide compound by using DFT/B3LYP/Cep-31G.
| Bond length (Å) | |||
| C1-N2 1.316 | C1-N3 1.369 | N3-C4 1.423 | N3-C5 1.423 |
| N2-C6 1.374 | C6-N7 1.317 | N7-C9 1.401 | C9-C10 1.412 |
| S8-C10 1.788 | C6-S8 1.796 | C10-C11 1.387 | C11-C12 1.396 |
| C9-C14 1.398 | C13-C14 1.393 | C12-C13 1.395 | |
| Bond angle (˚) | |||
| C4N3C5 121.22 | C1N2C6 120.34 | C6S8C10 87.96 | C1N3C4 118.69 |
| N2C6N7 120.39 | S8C10C9 108.95 | C1N3C5 120.08 | N2C6S8 123.37 |
| N7C9C10 116.47 | N3C1N2 119.79 | N7C6S8 116.23 | C12C13C14 121.12 |
| N7C9C14 124.65 | S8C10C11 128.24 | C10C11C12 117.08 | C11C12C13 121.27 |
| C9N7C6 110.39 | C9C10C11 122.81 | C13C14C9 118.84 | C10C9C14 118.88 |
| Dihedral angles (˚) | |||
| C4N3C1N2 179.99 | C1N2C6S8 0.00 | C6N7C9C10 0.00 | |
| C5N3C1N2 -0.00 | N2C6S8C10 -179.90 | S8C10C9C14 180.00 | |
| N3C1N2C6 179.90 | N2C6N7C9 179.90 | S8C10C11C12 -179.00 | |
| C1N2C6N7 -179.90 | C6N7C9C14 179.90 | N7C9C14C13 -179.00 | |
| N7C9C10C11 -179.90 | |||
| Charges | |||
| C1 0.247 | N2 -0.323 | N3 -0.095 | C4-0.043 |
| C5 -0.034 | C6 0.387 | N7 -0.306 | S8 -0.329 |
| C9 0.126 | C10 0.097 | ||
| Total energy/au | -114.844554 | ||
| Total dipole moment/D | 3.52 |
The accumulated charge on S8 (-0.329), C6 (0.387) and C10 (0.097), reflects that the S atom is bonded strongly with surrounded two carbon atoms. The presence of thiazole ring attached to the benzene ring effects on the charge spreading overall the compound, also the charges on the two terminal methyl group are effected by attached with nitrogen atom, the charge on C4 (-0.043) and C5 (-0.034). The energy of this compound is -114.844553978 au and very lower value of dipole 3.52D.
Pd2+ Complex with Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide Through The Nitrogen and Sulpher Atoms
In this complex, there are three plans one occupied by two terminal ends of the ligand bonded with Pd2+ through the nitrogen atoms of amino group and this plane is lying between others two planes occupied by two aromatic rings of the two ligand molecules as shown in Fig.(4). All parameters, selected inter atomic distances and angles of the complex are listed in Table (6). The complex is four-coordinate, the metal ion Pd2+ is coordinated to two unites of Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide molecules, one N-amino group and one S-thiazole ring of one Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide ligand. The Pd- S bond lengths (2.28Å and 2.27Å)34 are longer than the Pd-N (1.98Å and 1.99Å).35,36 The angles around central metal ion Pd2+ with surrounded four donating atoms of the two molecules of Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide vary from 97.90˚ to 119.21˚, these values not deviated slightly from these expected for tetrahedral structure.37 The distances between Pd-S and Pd-N and angles around the central metal ion are given in Table (6), these values are in agreement with experimental data.38
Table 6: Equilibrium geometric parameters bond lengths (Å), bond angles (˚), dihedral angles (˚) and charge density of the first complex by using DFT/B3LYP/Cep-31G.
| Bond length (Å) | |||
| Pd-S7 2.28 | Pd-N3 1.98 | Pd-S19 2.27 | Pd-N15 1.99 |
| C5-S7 1.76 | C5-N2 1.26 | N2-C29 1.27 | N3-C29 1.39 |
| N15-C16 1.39 | C16-N17 1.26 | C18-N17 1.26 | C18-S19 1.76 |
| C5-S7 1.76 | C5-N2 1.26 | N2-C29 1.27 | N3-C29 1.39 |
| Bond angle (˚) | |||
| N15-Pd-S19 97.90 | N15-Pd-N3 115.08 | N15-Pd-S7 119.21 | |
| N3-Pd-S19 119.21 | S7-Pd-S19 108.70 | N3-Pd-S7 97.91 | |
| Dihedral angles (˚) | |||
| N15-Pd-N3-C29 79.09 | N15-Pd-S7-C5 -65.74 | S19-Pd-S7-C5 -176.56 | |
| S19-Pd-N3-C29 -165.07 | N3-Pd-N15C16 79.08 | N3-Pd-S19-C18 -65.73 | |
| S7-Pd-S19-C18 -176.56 | S7-Pd-N15-C16 -165.08 | ||
| Charges | |||
| Pd 1.030 | N2 -0.234 | N3 0.018 | C5 0.429 |
| S7 -0.068 | C29 0.281 | N15 0.018 | N17 -0.234 |
| C16 0.281 | C18 0.429 | S19 -0.068 | |
| Total energy/au | -259.9069355 | ||
| Total dipole moment/D | 2.754 |
The complex is a highly sterically-hindered, the two units of Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide with Pd2+ in this complx occupied three plans they are not perpendicular to each other. This result can be confirmed from the values of the dihedral angle N15PdN3C29,79.09˚ and N15PdS7C5, -65.74˚ , where the values are neither zero nor 180˚, so the terminal end of the two amino groups bonded with metal ion not axially oriented from the ring and then out of plane occupied by other two aromatic rings. Also the two aromatic rings lying out of the plane occupied by metal ion bonded with terminal two amino groups, this observation is supported by the values of calculated dihedral angles: S19PdS7C5, -176.56˚ and S19PdN3C29, -165.07˚. Fig.(4), reveals the optimized geometrical structure of Pd2+-Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide.
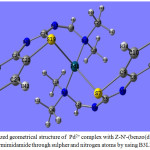 |
Figure 4: Optimized geometrical structure of Pd2+ complex with Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide through sulpher and nitrogen atoms by using B3LYP/Cep-31G. |
The charge accumulated on S7 and S19 are (-0.115 and -0.118 respectively) also, N3 and N15 are (0.027 and 0.009 respectively). The charge accumulated in N2 (-0.200) and N17 (-0.206) also the charge accumulated on carbon atoms, C5 and C29 are (-0.052 and 0.255) while C16 and C18 are (0.258 and 0.407) as shown in table (6). The energy of this complex is -259.906935547 au and dipole moment is 6.125 D.
Pd2+ Complex with Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide Through Two Nitrogen Atoms
The Pd2+ ion, at a crystallographic inversion center, is in a tetrahedral environment. In the axial plane the metal ion is coordinated by two nitrogen atoms (N-amino and N-thiazole) of two Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide ligands at the distances vary from 1.941 Å to 1.976 Å, these bond lengths are similar to those observed in related compounds.39 Fig.(5) reveals the optimized geometrical structure of the complex of Pd2+ with Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide through two nitrogen atoms with the atomic numbering scheme selected bond distances and angles are given in Table (7). The difference in the C18-N19 and C6-N8 (1.272 Å and 1.271Å) compared with the C6-N7 in the free ligand (1.317 Å ),40 confirms the formation of bond between the nitrogen atom of thiazole ring and Pd2+ ion; these bond lengths are virtually identical in uncomplexed Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide ligand. The tetrahedral coordination environment is detected from the values of angles around the Pd2+ in complex as given in Table (7) . The bond distance between Pd-N3 is 1.976 Å and Pd-N8 is 1.942 Å35 while the distance between Pd-N15 is 1.975Å and Pd-N19 is 1.941 Å.36 The Pd-N3 and Pd-N15 bond lengths are longer than that Pd-N8 and Pd-N19. The bond angles around the central metal ion Pd vary from 98.94˚ to 120.95˚; these values not differ significantly from these expected for tetrahedral structure. The energy of this complex is -260.013457522 au and the dipole moment is high 3.781D, so this complex is considered more stable.
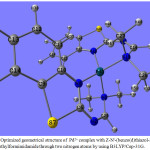 |
Figure 5: Optimized geometrical structure of Pd2+ complex with Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide through two nitrogen atoms by using B3LYP/Cep-31G. |
Table 7: Equilibrium geometric parameters bond lengths (Å), bond angles (˚), dihedral angles (˚) and charge density of the second complex by using DFT/B3LYP/Cep-31G.
| Bond length (Å) | |||
| Pd-N3 1.976 | Pd-N8 1.942 | Pd-N15 1.975 | Pd-N19 1.941 |
| C18-N17 1.263 | N19-C22 1.277 | N3-C29 1.394 | C29-N2 1.268 |
| N2-C6 1.263 | C6-N8 1.271 | C18-N19 1.272 | C6-S7 1.819 |
| N8-C10 1.277 | N15-C16 1.393 | C16-N17 1.268 | C18-S20 1.819 |
| Bond angle (˚) | N15-Pd-N8 111.56 | N15-Pd-N3 115.97 | |
| N15-Pd-N19 98.97 | N3-Pd-N19 111.45 | N3-Pd-N8 98.94 | |
| N8-Pd-N19 120.95 | |||
| Dihedral angles (˚) | |||
| N15-Pd-N8-C6 -96.13 | N15-Pd-N3-C29 81.21 | N19-Pd-N8-C6 148.23 | |
| N19-Pd-N3-C29 -166.59 | N3-Pd-N19C18 -96.08 | N3-Pd-N19-C22 79.97 | |
| N8-Pd-N15-C16 -166.61 | N8-Pd-N19-C18 148.42 | ||
| Charges | |||
| Pd 1.095 | N3 0.015 | N2 -0.259 | N8 -0.104 |
| C6 0.428 | C29 0.298 | N15 0.016 | C16 0.298 |
| N17 -0.259 | C18 0.429 | N19 -0.105 | |
| Total energy/au | -260.0134575 | ||
| Total dipole moment/D | 3.781 |
The bond distances between Pd2+ and surrounded nitrogen atoms of Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide in tetrahedral complex are shorter than that in other complex as shown in Tables (6 and 7). Also the charge accumulated on N-amino group, N3 and N15 are (0.015 and 0.016) and N-thiazole ring, N8 and N19 are (-0.104 and -0.105), in case of bonding of central metal ion with Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide through two nitrogen atoms. In case of other complex when Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide bonded with Pd2+ through sulpher atom of thiazole and one nitrogen atom of amino group,the charge on N-amino group, N3 and N15 are (0.017 and 0.019) and charge on S-thiazole S7 and S19 are -0.068. These values are indicating that there is a strong interaction between central metal ion Pd2+ which has charge equal +1.095 and more negative nitrogen atoms in second complex greater than that in first complex, at which Pd2+ has less positively charge (+1.03) in fist complex. The energy of the second complex is more negative than that in the first complex, moreover the dipole moment is high and the bond distances between the Pd2+ and surrounded four nitrogen atoms in the second complex are shorter than that in the first complex. These reasons have confirmed that the second tetrahedral complex is more stable than the first tetrahedral complex and Pd2+prefer coordinated with two molecules of Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide through two nitrogen atoms.
Frontier Molecular Orbitals
The frontier molecular orbitals play also an essential role in the UV-Vis. Spectra.41 Fig. 6, illustrates the distributions and energy levels of HOMO and LUMO orbitals computed for the all Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide and its complexes. For Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide compound the values of the energy of HOMO and LUMO are given in Table (8), the difference between HOMO and LUMO is 0.11586 for the free ligand, but in case of complexes the difference between HOMO and LUMO is 0.05134 for the first complex while in case of second complex 0.01782, the values of HOMO, LUMO, HOMO-1 , HOMO-2, LUMO+1 and LUMO+2 are given in Table (8).
 |
Figure 6: Molecular orbital surfaces and energy levels of Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide and its two complexes by using B3LYP/Cep-31G. |
Table 8: Values of energy (eV) of HOMO and LUMO for (A) Z-N’-(benzo(d)thiazol-2-yl)-N,N-dimethylformimidamide, (B) first complex and (C) second complexby using B3LYP/Cep-31G.
| (A) | (B) | (C) | |
| LUMO+2 | -0.15619 | -0.18169 | -0.19091 |
| LUMO+1 | -0.17839 | -0.19476 | -0.19233 |
| LUMO | -0.19618 | -0.19613 | -0.20865 |
| HOMO | -0.31204 | -0.24747 | -0.22647 |
| HOMO-1 | -0.33211 | -0.25493 | -0.22792 |
| HOMO-2 | -0.36322 | -0.25682 | -0.25851 |
| ∆E= HOMO – LUMO | 0.11586 | 0.05134 | 0.01782 |
Conclusion
The current method was utilized for the determination of palladium(II) in different water samples. The proposed procedures that involves coupling of BTDF as new reagent with palladium(II) for quantification of Pd(II) have some advantages such as selectivity, simplicity, stability and sensitivity as compared to many reported methods as provided in Table 4. Moreover, the methods is free from interference by different ions. The practical part are proved by theoretical studies.
References
- Joule, J. A.; Mills, K.; Smith, G.F. Heterocyclic Chemistry, 3rd ed., Chapman and Hall, London.1995.
- Davidson, J. P.; Faber, P. J.; Fischer, J. R.; Mansy, S.; Peresie, H. J.; Rosenberg, B.; VanCamp, L. Cancer Chemother. Rep.1975,59 (Pt 1), 287-300.
- Barton, J.; Rabinowitz, H.; Szalda, D.; Lippard, S. J. Am. Chem. Soc.1977,99, 2827-2829.
- Lippert, B. J. Clin. Hemat. Oncol. 1977, 7, 26-42.
- Lippert, B. Inorg. Chem. 1981, 20, 4326-4343.
- Chu, G.Y.H.; Duncan, R.E.; Tobias, R.S. Inorg. Chem. 1997.36. 2625.
- Lippert, B. in: Lippert B. (Ed.), Cisplatin, VHCA and Wiley-VCH, Zu¨rich and Weinheim,1999, pp. 379–403.
- Sakaguchi, S.; Kawakami, M.; O’Neill, J.; Yoo, K. S.; Jung, K. W.J. Organomet. Chem.2010,695, 195-200.
- Salas, J. M.; Romero, M. A.; Sánchez, M. P.; Quirós, M.Coord. Chem. Rev. 1999,193, 1119-1142.
- Falvello, L.; Forniés, J.; Martin, A.; Navarro, R.; Sicilia, V.; Villarroya, P.Chem. Commun. 1998, 2429-2430.
- Deacon, G. B.; Delbridge, E. E.; Forsyth, C. M.; Skelton, B. W.; White, A. H. J. Chem. Soc. Dalton Trans.. 2000, 745-751.
- Gust, K. R.; Knox, J. E.; Heeg, M. J.; Schlegel, H. B.; Winter, C. H.Angew. Chem. Int. Ed. 2002,114, 1661-1664.
- Deacon, G. B.; Forsyth, C. M.; Gitlits, A.; Harika, R.; Junk, P. C.; Skelton, B. W.; White, A. H.Angew. Chem. Int. Ed. 2002,114, 3383-3385.
- Perera, J. R.; Heeg, M. J.; Schlegel, H. B.; Winter, C. H.J. Am. Chem. Soc.1999,121, 4536-4537.
- (a) Lumme, P.; Mutikainen, I.; Lindell, E.Inorg. Chim. Acta. 1983,71, 217-226; (b) Goher, M. A.; Mautner, F. A.; Abu-Youssef, M. A.Transit Metal Chem. 1999,24 (1), 29-34.(c) Sakai, K.; Tomita, Y.; Ue, T.; Goshima, K.; Ohminato, M.; Tsubomura, T.; Matsumoto, K.; Ohmura, K.; Kawakami, K.Inorg. Chim. Acta. 2000,297, 64-71.
- Okano, T.; Uekawa, T.; Morishima, N.; Eguchi, S.J. Org. Chem. 1991,56, 5259-5262.
- (a) Umada, A.; Okano, T.; Eguchi, S.Synthesis. 1994, 1457-1462. (b) Gerus, I.; Gorbunova, M.; Vdovenko, S.; Yagupol’skii, Y. L.; Kukhar’, V.Chem. Inform 1991,22, 1878.
- Plancquaert, M.A.; Redon, M.; Janousek, Z.; Viehe, H. G.Tetrahedron.1996,52, 4383-4396.
- Tanaka, K.; Maeno, S.; Mitsuhashi, K. Heterocycl Chem.1985,22, 565-568.
- Livshits, N.; Valiev, G.; Khasanov, S.; Kadyrov, C. S.Chem. Natural Compd.1982,18, 762-763.
- Britton, H. T. S. Hydrogen Ions, fourth ed, Chapman and Hall, London, 1952, p. 113.
- Ruhela, R.; Sharma, J.; Tomar, B.; Hubli, R.; Suri, A.Talanta. 2011,85, 1217-1220.
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Zakrzewski, V.; Montgomery Jr, J.; Stratmann, R.; Burant, Dapprich, J. S. Millam, J.M. Daniels, A.D. Kudin, K.N. Strain, M.C. Farkas, O. Tomasi, J. Barone, V. Cossi, M. Cammi, R. Mennucci, B. Pomelli, C. Adamo, C. Clifford, S.Ochterski, Petersson, J. G. A. Ayala, P. Y. Cui, Q. Morokuma, K. Malick, D. K. Rabuck, A.D. Raghavachari, K. Foresman, J.B. Cioslowski, J. Ortiz, J.V. Stefanov, B.B. Liu, G. Liashenko, A. Piskorz, P. Komaromi, Gomperts,I. R.Martin, R. L. Fox, D. J. Keith, T. Al-Laham, M. A. Peng, C.Y. Nanayakkara, A. Gonzalez, C. Challacombe, M. P. Gill, M. W. Johnson, B. Chen, W. Wong, M. W. Andres, J. L. Gonzalez, C. Head-Gordon, M. Replogle, E. S. and Pople, J. A. Gaussian 98, revision A. 6; Gaussian, Inc. Pittsburgh, Pa 1998,40.
- Stevens, W. J.; Krauss, M.; Basch, H.; Jasien, P. G.Can. J. Chem.1992,70, 612-630.
- Kohn, W.; Sham, L. J.Phys. Rev.1965,140, A1133.
- Becke, A. D. Phys. Rev. A. 1988,38, 3098.
- Lee, C.; Yang, W.; Parr, R. G.Phys. Rev B.1988,37, 785.
- Flurry R. L., Molecular Orbital Theory of Bonding in Organic Molecules, Marcel Dekker. New York, 1968.
- (a) Masciocchi, N.; Moret, M.; Cairati, P.; Sironi, A.; Ardizzoia, G. A.; La Monica, G. J. J.o. t. A. C. S.J. Am. Chem. Soc. 1994,116, 7668-7676; (b) Masciocchi, N.; Ardizzoia, G. A.; Maspero, A.; LaMonica, G.; Sironi, A.Inorg. Chem.1999, 38, 3657-3664; (c) Ehlert, M.; Rettig, S.; Storr, A.; Thompson, R.; Trotter, J.Can. J. Chem.1989, 67, 1970-1974. (d) Murray, H.; Raptis, R. G.; Fackler Jr, J. P.Inorg. Chem. 1988,27, 26-33.
- Patent, Schenley Ind., US 2719161, 1952.
- Turel, I.; Bukovec, P.; Quirós, M.Int. J. Pharm.1997,152, 59-65.
- Sartorelli, A. C.; Agrawal, K. C.; Tsiftsoglou, A. S.; Moore, E. C.Adv. Enzyme Regul.1977,15, 117-139.
- Ali, M. A.; Mirza, A. H.; Butcher, R. J.; Tarafder, M.; Keat, T. B.; Ali, A. M.J. Inorg. Biochem.2002,92, 141-148.
- Xie, Y.-B.; Li, J.-R.; Zheng, Y.; Bu, X.-H.; Zhang, R.-H.J. Mol. Struct.2003,645, 227-230.
- Sakaguchi, S.; Kawakami, M.; O’Neill, J.; Yoo, K. S.; Jung, K. W.J. Organomet. Chem.2010,695, 195-200.
- Chakraborty, J.; Saha, M. K.; Banerjee, P.Inorg. Chem. Commun.2007,10, 671-676.
- Montoya, V.; Pons, J.; Garcia-Antón, J.; Solans, X.; Font-Bardia, M.; Ros, J.InorganicaChim. Acta.2007,360, 625-637.
- Serrano, J. L.; Garcı́a, L.; Pérez, J.; Pérez, E.; Vives, J.; Sánchez, G.; López, G.; Molins, E.; Orpen, A. G.Polyhedron.21, 1589-1596.
- (a) Philp, D.; Stoddart, J. F.Angew. Chem. 1996,35, 1154-1196.
- Lehn, J.-M.; Sanders, J.Angew. Chem.1995,34, 2563.
- Shen, L. L.; Hopper, D. C.; Wolfson, J. S. (Eds.), Quinolone Antimicrobial Agents, Second ed., A.S.M. Washington, DC, 1993, P.77.
- Fleming,I., Frontier Orbitals and Organic Chemical Reactions, Wiley, London, 1976.

This work is licensed under a Creative Commons Attribution 4.0 International License.









