Hydrocracking of LDPE Plastic Waste Into Liquid Fuel over Sulfated Zirconia from a Commercial Zirconia Nanopowder
Latifah Hauli, Karna Wijaya* and Akhmad Syoufian
and Akhmad Syoufian
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Gadjah Mada, Sekip Utara Bulaksumur, Yogyakarta, 55281, Indonesia.
Corresponding Author E-mail: karnawijaya@ugm.ac.id
DOI : http://dx.doi.org/10.13005/ojc/350113
Article Received on : 04-12-2018
Article Accepted on : 06-01-2019
Article Published : 14 Feb 2019
Hydrocracking of LDPE plastic waste into liquid fuel was conducted by using sulfated zirconia (SZ) and Cr metal supported on SZ (Cr/SZ) catalysts. Catalysts were prepared and characterized by SEM-EDS, SAA, and TGA-DTA. The liquid produced was characterized by GC-MS. The characterization result showed the sulfate group was successfully impregnated on ZrO2 nano powder with the higher content of S and O than ZrO2 and then decreased after addition of Cr metal as confirmed in SEM-EDS result. All catalysts were in mesoporous material as observed by SAA characterization and also showed the highest specific surface area of Cr/SZ catalyst of 14.56 m2g-1. The catalytic activity increased by the presence of Cr on SZ with liquid produced about 40.15%. Hydrocracking process also increased the gasoline fraction and the highest gasoline fraction was produced by Cr/SZ catalyst about 89.91%.
KEYWORDS:Catalyst; Fuel; LDPE Plastic; Sulfated Zirconia
Download this article as:| Copy the following to cite this article: Hauli L, Wijaya K, Syoufian A. Hydrocracking of LDPE Plastic Waste Into Liquid Fuel over Sulfated Zirconia from a Commercial Zirconia Nanopowder. Orient J Chem 2019;35(1). |
| Copy the following to cite this URL: Hauli L, Wijaya K, Syoufian A. Hydrocracking of LDPE Plastic Waste Into Liquid Fuel over Sulfated Zirconia from a Commercial Zirconia Nanopowder. Orient J Chem 2019;35(1). Available from: https://bit.ly/2UTFrpO |
Introduction
Plastic was polymeric compound that was very much found in the activities of human life so that the increasing amount of plastic used also had a significant impact in the increasing of plastic waste produced.1,2 Plastic waste which accumulated could cause the environmental problems and became a threat because the plastic waste was very difficult to disintegrate in the soil and required a very long time to be degraded.3,4 Hydrocracking was one of the most promising and highly developed technique to break plastic polymer. This process could be solutions to overcome the problem of plastic waste and also the lack of the fuel availability because it was potential to crack the plastic into liquid fuel.5,6 It also usually began with the pyrolysis as initial step.7
In hydrocracking, heterogeneous catalyst was more widely used and developed because of their high thermal stability, safe used, selectivity to the product, environmentally friendly, and reusable. Heterogeneous catalyst that was currently being developed to convert polymers was the groups of metal oxides such as zirconia (ZrO2). ZrO2 had the good characteristic i.e. the corrosion resistant, high thermal stability, low thermal conductivity so that material was very potential to be used in many applications.8–12
Modification of ZrO2 material with the strong acid such as sulfate acid had proved an increasing of the surface acidity and activity.13 Several methods for preparation of SZ catalyst had also been successfully investigated could increase of acidity.14–16 In this study, the wet impregnation method was conducted to get the SZ catalyst from a commercial ZrO2 nanopowder then Cr metal was impregnated to SZ by reflux method. The catalyst obtained was tested in hydrocracking process of low-density polyethylene (LDPE) plastic waste in application. Several catalytic properties of those catalysts, the yield conversion, and the selectivity of the liquid produced also were learned in this research. The used of the synthesis method for these catalysts and development of the catalysts test in plastic conversion to liquid fuel had never been done before.
Experimental
Materials
Commercial zirconia nanopowder was supplied from China (purity 99%). Sulfuric acid (98%), ammonia (25%), and chromium (III) nitrate nonahydrate was obtained from Merck. The low density polyethylene (LDPE) plastics were collected from the final disposal in Yogyakarta. Hydrogen gas was supplied by PT. Samator Gas Industri.
Preparation of SZ Catalyst
Sulfated zirconia catalyst was prepared by wet impregnation method. ZrO2 nano powder about 10 g was added into 150 mL 0.8 M H2SO4 solution then stirred for 24 h and calcined for 4 h at 600°C.14 SZ catalyst which obtained then dried in an oven at 100°C for overnight. Catalyst was crushed and sieved in a 250 mesh sieve. Catalyst produced was labeled with SZ.
Chromium (Cr) metal was impregnated into SZ catalyst by wet impregnation method using salt of chromium (III) nitrate nonahydrate (1.0% (w/w)), dissolved in 100 mL of distilled water and added SZ catalyst, then refluxed at 90°C for 4 h. Catalysts were dried in the oven at 100°C for overnight, calcined at 600°C, then reduced by flowing H2 gas at 400°C for 2 h with a flow rate of 10 mL/min. Catalyst produced was labeled with Cr/SZ. The acidity of all catalysts was determined by gravimetric method with ammonia adsorption.
Hydrocracking Experiment
The plastic raw material was cleaned by washing, dried then cut into small pieces. Plastic was heated at 300-400°C for 4 h in the pyrolysis reactor. The gas produced in the pyrolysis reactor was condensed into a liquid phase through a condenser. The liquid produced from pyrolysis was hydrocracked at 300°C for 1 h under H2 gas stream with a flow rate of 10 mL/min. The hydrocracking process was conducted in the hydrocracking microreactor by using the catalyst with the feed/catalyst ratio of 100. The conversion yield was calculated by using the equations:
The liquid product from pyrolysis and hydrocracking of LDPE plastic waste was characterized by GC-MS.
Instrumentation
The surface morphology of ZrO2and SZ catalysts was investigated by scanning electron microscope (SEM, JEOL JSM-6510) which equipped with the energy dispersive spectrometer (EDS, JED-2300) for detecting the elements of these catalysts. The specific surface area, pore volume, and pore diameter were characterized by surface area analyzer (SAA, Quantachrome NovaWin Series version 11.0). The sample was outgassed for 4 h at 200°C. The physical adsorption of N2 gas was conducted at batch temperature 77.3 K.
The surface parameter such as specific surface area was calculated by using the Brunauer–Emmett–Teller (BET) method while the average pore diameter was calculated by using the Barrett-Joyner-Halenda (BJH) method. Acidity of catalysts was identified by fourier transform infrared spectroscopy (FTIR, Shimadzu Prestige-21) with a KBr disc technique in range 4000-500 cm-1. Thermogravimetric analysis (TGA) and differential thermal analysis (DTA) for SZ catalyst were also conducted by using DTG-60 instrument which operated range from room temperature to 600°C in air with flow rate 30 mL/min. The liquid product was analyzed by using gas chromatography-mass spectrometer (GC-MS, Shimadzu QP 2010S) with the column length about 30 m and helium as a carrier gas.
Results and Discussion
SEM-EDS Characterization
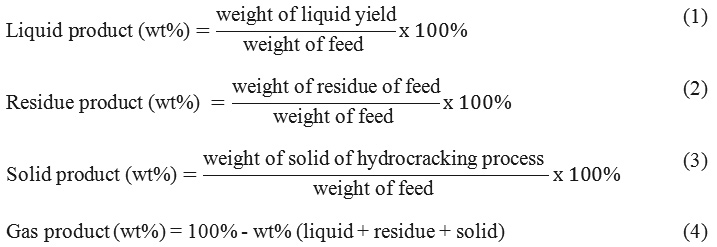
Fig.1 showed the morphology images of ZrO2 nano powder and SZ catalysts which observed by SEM. SEM result indicated the different morphology surface feature from before and after addition of sulfate. The presence of agglomeration particle occurred after sulfate addition as shown in Fig.1. SEM-EDS could not identify Cr particles. However, it observed the different size and disordered shape of all catalysts. The elements of Zr, O, and S in the catalysts which observed by EDS were presented in Table 1. EDS result showed the content of O and S increased after addition of sulfate and decreased after impregnating of Cr metal. It indicated that ZrO2 was successfully impregnated by sulfate and Cr metal.17
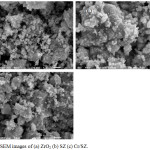 |
Figure 1: SEM images of (a) ZrO2 (b) SZ (c) Cr/SZ. |
Table 1: Elements analysis (EDS) of catalysts.
|
Sample |
Mass (%) |
||
|
Zr |
O |
S |
|
| ZrO2 |
71.33 |
28.06 |
0.54 |
| SZ |
67.47 |
30.41 |
2.09 |
| Cr/SZ |
70.75 |
27.79 |
0.54 |
SAA Characterization
The specific surface area, average pore diameter, and total pore volume of catalysts were presented in Table 2. The specific surface area of SZ was smaller than ZrO2 nanopowder. This decreasing occurred because the sulfate group loaded into the pores of ZrO2 nano powder at impregnation process.14 Possibility of surface area reduction also caused by sulfate group which was not evenly distributed to the surface and formed agglomeration with ZrO2.18 Specific surface area increased after addition of Cr metal on SZ catalyst. This occurred because Cr metal was evenly distributed on the surface of catalyst. There were no significant changes from the total pore volume and the average pore diameter. The presence of sulfate which possibly entered the pore could slightly widen the pore size and thus increased the total pore volume. The sulfate group which did not fill the small pores could make the overall average pore diameter would be small.
Fig. 2 showed the isotherm adsorption-desorption of N2 gas. In this study, all catalysts were in type IV isotherm which essentially indicated mesoporous material (between 25 and 55.5 Å) as according to IUPAC classification. It also confirmed by average pore diameter as shown in Table 2. Sing et al.,19 had reported that type IV isotherm was indicated the adsorption from monolayer to multilayer. Mesoporous material characteristic of was the hysteresis loop which attributed to the capillary condensation. In this study, ZrO2 and Cr/SZ catalysts had the hysteresis of type H4 loop while SZ catalyst had the hysteresis of type H3 loop. Type H3 loop, it seem likely did not show the limiting adsorption at high P/P0. It was associated with the plate-like particle aggregates that created the slit-shaped pores. Type H4 loop was attributed to the narrow slit-like pore.
Table 2: Textural properties of catalysts.
|
Sample |
SBET (m2g-1) |
Average pore diameter (Å) |
Total pore volume (cm3g-1) |
| ZrO2 |
12.27 |
36.98 |
0.07 |
| SZ |
7.79 |
36.84 |
0.08 |
| Cr/SZ |
14.56 |
36.96 |
0.07 |
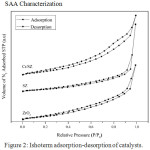 |
Figure 2: Ishoterm adsorption-desorption of catalysts. |
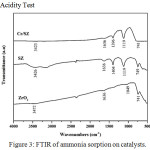 |
Figure 3: FTIR of ammonia sorption on catalysts. |
Table 3: Acidity value of catalyst.
|
Sample |
Acidity value (mmol/g) |
| ZrO2 |
0.06 |
| SZ |
3.81 |
| Cr/SZ |
8.22 |
FTIR spectra of ammonia sorption on ZrO2, SZ, and Cr/SZ catalysts was presented in Fig. 3. Ammonia adsorbed on Brønsted acid sites was characterized with vibration band at 1404 cm-1 while the ammonia adsorbed on Lewis acid sites was detected around at 1119 cm-1.21–22 The presence of both Brønsted and Lewis acid sites could increase acidity of catalysts.23 It also was investigated from acidity value in Table 3. Acidity of SZ was higher than ZrO2, it indicated that sulfate group was successfully impregnated on ZrO2.14 Acidity of Cr/SZ catalyst was highest, it indicated that Cr metal was successfully impregnated on SZ catalyst. It was confirmed from acidity value of Cr/SZ catalyst about 8.22 mmol/g. Lewis acid sites was available from the empty d-orbital of Cr metal which it could receive free electron pair from nitrogen of ammonia. Because of it, acidity value of Cr/SZ catalyst was so high.
Thermal Analysis Characterization
TGA and DTA of SZ catalyst were presented in Fig.4. TGA showed the weight loss above 200°C (0.82 mg). It attributed to dehydration of absorbed water which also was showed at DTA characterization as endotherm process.24 Amalia et al.,25 had reported that decomposition of sulfate could occur at the high temperature from 873-1213 K. Hiromi et al.,26 also had reported that decomposition of surface sulfate began to occur above 773 K and the weight would decrease gradually until 1273 K. In this study, the decomposition of sulfate had not been detected because of the short temperature range used. The result showed that the material was stable up to 600°C.11 That result also appeared another endothermic low intensity peak around at 340°C as shown at DTA characterization and could be attributed to dehydroxylation process.27
 |
Figure 4: TGA and DTA of SZ catalyst. |
Catalysts Activity
The activity of ZrO2, SZ, and Cr/SZ catalysts was conducted for hydrocracking of LDPE plastic waste. Pyrolysis process of LDPE plastic waste into liquid was conducted as the first step to prepare feed then was continued with a catalytic hydrocracking process to get results with a fraction that was lighter than the liquid fuel produced previously.28 The conversion of LDPE plastic waste at temperature of 300°C was showed in Fig.5. The hydrocracking of LDPE plastic waste produced gas, liquid, residue, and solid yield. The highest liquid yield was produced by Cr/SZ catalyst of 40.15% while the highest gas and residue yields were produced by ZrO2 catalyst of 38.07% and 44.69%, respectively. One of the factor could influence catalytic activity was an acidity of the catalyst which increased the presence of the Lewis and Brønsted acid sites.23 It had been previously mentioned that the presence of d-orbital of Cr metal which could increase the acidity and make it easy to bind with radical hydrogen from homolysis reaction of hydrogen gas in hydrocracking process. The wider surface area of Cr/SZ catalyst also made it easier to interact with much reactant which could break the macromolecules of plastic polymers on the external surface of catalyst through a carbonium ion mechanism.29 It caused the activity of Cr/SZ catalyst became greater and also affected the amount of liquid produced. It was proven that Cr/SZ catalyst could increase the liquid yield in hydrocracking experiment.
The high temperature of hydrocracking process could cause the formation of radical compounds due to the reaction of homolysis which these radical compounds produced could bind to the other radical compounds then formed the short-chain carbon compounds. This compound usually was in the gas phase.30 Therefore, in this study also still produced a lot of gas yields. Residue yield from hydrocracking process for all catalysts was still much. However, the used of Cr metal supported on SZ catalysts could reduce residue yield. The result also showed that all catalyst produced the solid yield. The contact of catalyst with hydrocarbons at high temperatures could form coke deposits on the surface and loss of sulfate group.20 This caused Cr/SZ catalyst had the highest solid yield.
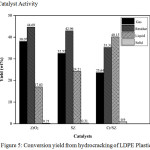 |
Figure 5: Conversion yield from hydrocracking of LDPE Plastic. |
Selectivity of liquid yield from pyrolysis and hydrocracking process also presented in this research. The selectivity of pyrolysis product was presented in Fig. 6. The result showed that the highest selectivity from pyrolysis process was in diesel fraction (C13-C20) of 52.27%. Pyrolysis process also still produced heavy fraction (C>20) of 0.41%. Fig.8 showed the selectivity of liquid product from catalytic hydrocracking process. The result showed the decreasing of diesel fraction and the increasing gasoline fraction after hydrocracking with ZrO2, SZ, and Cr/SZ catalysts. The heavy fraction also did not appear in this result. Based on the selectivity result, catalytic hydrocracking process produced the high gasoline fraction as desired.
Fig.7 also exhibited the highest gasoline fraction selectivity was produced by Cr/SZ catalyst about 89.91%. This result also proved that the presence of Cr metal on SZ catalyst increased the liquid product from hydrocracking of LDPE plastic waste and also selectivity for gasoline fraction produced.
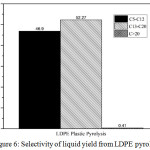 |
Figure 6: Selectivity of liquid yield from LDPE pyrolysis. |
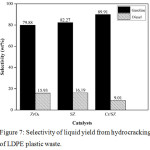 |
Figure 7: Selectivity of liquid yield from hydrocracking of LDPE plastic waste. |
Conclusion
Sulfate group was successfully impregnated on ZrO2 nanopowder with the higher content of S and O than ZrO2catalyst and decreased after addition of Cr metal as confirmed in SEM-EDS result. All catalysts was in mesoporous material as observed by SAA characterization and also showed the highest specific surface area of Cr/SZ catalyst of 14.56 m2g-1. Hydrocracking of LPDE plastic waste produced the highest liquid product which conducted by using Cr/SZ catalyst about 40.15% and it could reduce residue and gas yield. Based on selectivity, the pyrolysis process still produced the high diesel fraction in the liquid yield and the gasoline fraction increased after hydrocracking process from using all catalysts. The highest gasoline fraction was produced by Cr/SZ catalyst about 89.91%.
Acknowledgments
This work was financially supported by Indonesia Ministry of Research, Technology, and Higher Education under program of PMDSU Batch II (Contract number: 2028/UN1/DITLIT/DIT-LIT/LT/2018).
Conflict of Interest
There is no conflict of interest.
References
- Hazrat, M. A.; Rasul, M.G.; Khan, M. M. K. Procedia Eng. 2015, 105, 865-876.
- Buekens, A.; Huang, H. Resour Conserv Recycl. 1998, 23, 163-181.
- Sarker, M.; Rashid, M. M.; Rahman, M. S.; Molla, M. J Environ Prot. 2012, 03, 700-708.
- Hamid, S.H.; Amin, M. B. J. Appl Polym Sci. 1995, 55, 1385-1394.
- Demirbas, A. J. Anal Appl Pyrolysis. 2004, 72, 97-102.
- Kunwar, B.; Cheng, H. N.; Chandrashekaran, S. R.; Sharma, B. K. Renew Sustain Energy Rev. 2016, 54, 421-428.
- Syamsiro, M.; Saptoadi, H.; Norsujianto, T. Energy Procedia. 2014, 47, 180-188
- Siddiqui, M. R. H.; Al-wassil, A. I.; Al-Otaibi, A. M.; Mahfouz, R. M. Mater Res. 2012, 15, 986-989.
- Sivasamy, A.; Cheah, K. Y.; Fornasiero, P.; Kemausuor, F.; Zinoviev, S.; Miertus, S. Chem Sustain Energy Mater. 2009, 2, 278-300.
- Fatimah, I.; Rubiyanto, D.; Kartika, N. C. Indones. J. Chem. 2016, 16, 8-13.
- Patel, A.; Brahmkhatri, V.; Singh, N. Renew Energy. 2013, 51, 227-233.
- Raissi, S.; Kamoun, N.; Younes, M. K.; Ghorbel, A. React Kinet Mech Catal. 2015, 115, 499-512.
- Jin, T.; Yamaguchi, T.; Tanabe, K. J. Phys Chem. 1986, 90, 4194-4196.
- Hauli, L.; Wijaya, K.; Armunanto, R. Orient. J. Chem. 2018, 34, 1559-1564.
- Yu, S.; Jiang, P.; Dong, Y.; Zhang, P.; Zhang, Y.; Zhang, W. Bull. Korean Chem. Soc. 2012, 33, 524-528.
- Li, B.; Gonzalez, R. D. Ind. Eng. Chem. Res. 1996, 35, 3141-3148.
- Utami, M.; Wijaya, K.; Trisunaryanti, W. Key Eng. Mater. 2017, 757, 131-137.
- Utami, M.; Wijaya, K.; Trisunaryanti, W. Mater. Chem. Phys. 2018, 213, 548-555.
- Sing, K. S. W.; Everett, D. H.; Haul, R. A. W.; Moscou, L.; Pierotti, R. A.; Rouquerol, J.; Siemieniewska, T. J. Pure Appl. Chem. 1985, 57, 603-619.
- Aboul-gheit, A. K.; Gad, F. K.; Abdel-aleem, G. M.; El-desouki, D. S. Egypt J Pet. 2014, 23, 303-314.
- Sohn, J. R.; Kwon, T. D.; Kim, S. B.. Bull. Korean Chem. Soc. 2001, 22, 1309-1315.
- Jiang, K.; Tong, D.; Tang, J.; Song, R.; Hu, C. Applied Catal. A. Gen. 2010, 389, 46-51.
- Kustov, L. M.; Kazansky, V. B.; Figueras, F.; Tichit, D. J. Catal. 1994, 150, 143-149.
- Srinivasan, R.; Keogh, R. A. J. Catal. 1995, 153, 123-130.
- Luz, A.; Pereira, C.; Gustavo, S.; Albornoz, A. Applied Catal. A. 2008, 334, 187-198.
- Matsuhashi, H.; Nakamura, H.; Ishihara T,; Iwamoto, S.; Kamiya, Y. Applied Catal. A. 2009, 360, 89-97.
- Aboul-Gheit, A. K.; El-Desouki, D. S.; Abdel-Hamid, S. M.; Ghoneim, A.; Ibrahim, A. H.; Gad, F. K. Egypt J. Chem. 2012, 527, 509-527.
- Sriningsih, W.; Saerodji, M. G.; Trisunaryanti, W.; Armunanto, R.; Falah, I. Procedia Environ Sci. 2014, 20, 215-224.
- Qader, S. A.; Hill, G. R. IEC Process Des Dev. 1969, 8, 456-461.
- Pongsendana, M.; Trisunaryanti, W.; Artanti, F. W.; Falah, I. Korean J. Chem. Eng. 2017, 34, 2591-2596.

This work is licensed under a Creative Commons Attribution 4.0 International License.









