Graphene Nanosheets Effect to Improve CO-Tolerance of Pt/Graphene Nanosheets Catalyst
Kerista Sebayang1, Rikson Siburian2 and Minto Supeno2
and Minto Supeno2
1Department of Physics, Faculty of Mathematics and Natural Sciences, University of Sumatera Utara, Medan-Indonesia.
2Department of Chemistry, Faculty of Mathematics and Natural Sciences, University of Sumatera Utara, Medan-Indonesia.
Corresponding Author E-mail: riksonsiburian2000@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/340618
Article Received on : 17-10-2018
Article Accepted on : 18-11-2018
Article Published : 18 Nov 2018
In this paper, we concern about CO tolerance of Platinum (Pt)/Graphene Nanosheets (GNS) catalysts dependence of pH. The aim of this research is to investigate relationship between CO tolerance and Pt particle size base on pH dependence and also supporting material effect to CO-tolerance. The research method is modified Hummers and impregnation method to synthesize GNS and Pt/GNS catalysts, respectively. The results show that electrocatalytic measurements of Pt/GNS catalyst prepared at pH = 1 has the highest CO tolerance (69.4 %) under 500 ppm CO level in the hydrogen oxidation reaction among pH = 6, 12.5 and Pt/CB commercial catalyst. It means pH dependence and GNS may be expected to enhance electrocatalytic activity (CO tolerance) of Pt. Furthermore, GNS is possible used as an anode catalyst of Polymer Electrolyte Fuel Cells (PEFCs).
KEYWORDS:CO Tolerance; Electrocatalytic; Graphene Nano Sheets; pH; Pt/GNS
Download this article as:| Copy the following to cite this article: Sebayang K, Siburian R, Supeno M. Graphene Nanosheets Effect to Improve CO-Tolerance of Pt/Graphene Nanosheets Catalyst. Orient J Chem 2018;34(6). |
| Copy the following to cite this URL: Sebayang K, Siburian R, Supeno M. Graphene Nanosheets Effect to Improve CO-Tolerance of Pt/Graphene Nanosheets Catalyst. Orient J Chem 2018;34(6). Available from: http://www.orientjchem.org/?p=52807 |
Introduction
Recently, many researchers desire to increase the CO tolerance of electrode catalyst. It may be expected to improve the performance of hydrogen-proton exchange membrane fuel cells Hydrogen- Polymer Electrolyte Fuel Cells (H2-PEMFC). Pt nanoparticles are believed be able to improve the catalytic activity at the anode catalyst of H2-PEMFC system.1 Hydrogen as a fuel for H2-PEMFC, it was produced by reforming hydrocarbon. Unfortunately, CO exists on hydrogen. In presence of CO, Pt surface may be blocked on it, causing Pt deactivated (CO poison).2-5 Many researchers have tried to improve CO tolerance. Ticianelli (2013) concluded that CO tolerance of PtMo/C was higher than Pt/C.6 The stability of anode may be improved by using Pt/MoO2-C.7 Pt alloy catalysts also have been recognized in order to increase the catalytic activity of fuel cells.8 The size of Pt particles may affect the catalyzing CO9 and NO oxidation.10 On the other hand, supporting materials are also a pivotal point to develop economically and durable fuel cells. There are many types of supporting materials which were reported to improve electrocatalytic activity as a supporting material for Pt and its alloy, such as Ti0.7W0.3O2,11 carbon black,3,12-15 CNT,16-20 Ti4O7,21 CNF,22 carbon xerogel,23 graphene,24-27 graphite,28,29 N-graphene,30-32 and GNS.2,33-36
On the previous research, we found that GNS may role to control the Pt particle size37 and proposed the formation process of Pt subnano-cluster on GNS.38 Interestingly, the GNS may affect the electronic structure of Pt.39 Unfortunately, we have not clarified the CO tolerance of Pt/GNS. In this paper, we reported the GNS roles as a support material and also pH dependence for Pt particle size as an electrode catalyst on H2-PEMFC especially for CO tolerance. We expect that CO tolerance may be enhancing by using Pt/GNS catalyst and GNS may be expected to enhance electrocatalytic activity of Pt as a candidate for anode catalyst of H2-PEMFC.
Materials and Methods
GNS were obtained via a solution-based route, involving the chemical oxidation of graphite to be hydrophilic graphite oxide. Graphite oxides were exfoliated to form individual graphene oxide sheets by ultra-sonication in water.40 Then, it was reduced tobe graphene nanosheets.41,42 The 30 wt % Pt catalysts were deposited onto GNS using a platinum (Pt) precursor of [H2PtCl6.6H2O] (Alfa Aesar, A Johnson Matthey Company). Briefly, the ethanol solution of the precursor was mixed with the ethanol solution of GNS. Then, pH of the mixed solution was adjusted to be pH (1; 6 and 12.5), respectively by dropping 1 M NaOH aqueous. This solution was stirred for 3 hours. We used 3 hours due to all of solution has been well mixed each other and pH value is constant. The end of 3 hours, the product was collected by filtration and dried in air at 60oC for 12 h. Then, it was subjected to heat treatment in a hydrogen stream at 400oC for 2h in a furnace.37,38 GNS and the effect of pH for Pt/GNS catalysts were characterized by X-ray diffraction (XRD) (Philips X’pert Pro X-ray diffractometer with Cu-Kα radiation of 1.541 Å), Transmission electron microscope (TEM) (JEOL JEM-1400), and electrochemical measurement (PGSTAT PG12, AUTO LAB Potentiostat/Galvanostat), respectively.
Result and Discussion
Characterization of GNS
Firstly, GNS was characterized by using XRD and TEM. XRD pattern of GNS formation may be seen in Figure 1.
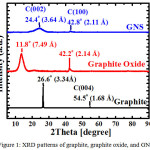 |
Figure 1: XRD patterns of graphite, graphite oxide, and GNS.
|
It clearly express that graphite was oxidized to form graphite oxide and finally, it was reduced by using hydrazine to generate GNS (Figure 1). The C (002) peak of GNS is very weak and broad, indicating graphene sheets are not stacking. It is totally different with the graphite peak, meaning graphene stacking has been reduced be GNS. In addition, oxidation of graphite was succeeding to produce graphite oxide, indicating the sharp peak was appear at 2θ = 11.8o. Base on XRD data, we may conclude that GNS may be generated from graphite as well as a raw material.
In order to probe that GNS was formed, we also analyzed it by using TEM. TEM image of GNS is shown in Figure 2.
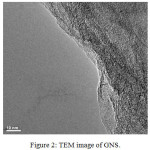 |
Figure 2: TEM image of GNS.
|
GNS surface is very thin, flat and no coalesce. Its sheets are very clear. Therefore, we believe that GNS was formed.
CO Tolerance of Pt/GNS
The electro catalytic activity of Pt 30 wt %/GNS at pH = 1, 6 and 12.5 and Pt 30 wt %/CB (Johnson Matthey commercial catalyst) are shown in Figure 3 and 4, respectively.
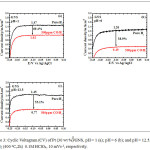 |
Figure 3: Cyclic Voltagram (CV) of Pt (30 wt %)/GNS, pH=1 (a); pH=6 (b); and pH=12.5, red.H2 (400oC,2h) 0.1M HClO4, 10 mVs-1, respectively.
|
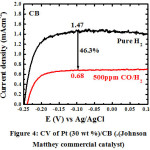 |
Figure 4: CV of Pt (30 wt %)/CB (.(Johnson Matthey commercial catalyst).
|
Figure 3 shows the effect of pH on Pt/GNS catalysts. The Pt/GNS (pH = 1) shows CO tolerance is highest among pH = 6 and 12.5. Interestingly, CO tolerance of Pt/GNS at pH = 1 is also higher than Pt/CB (commercial catalyst) (Figure 4). CO tolerance of Pt/GNS at pH = 1 is highest due to two effects, those are: enthalpy and entropy factors.37,38 Enthalpy factor occurs because GNS has π-conjugated system (Figure 5). It may cause interaction chemically between Pt and GNS via the π-d hybridization (Figure 6).39,40
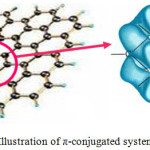 |
Figure 5: Illustration of π-conjugated system of GNS.
|
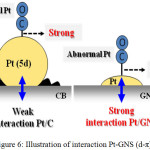 |
Figure 6: Illustration of interaction Pt-GNS (d-π).
|
Figure 6 describes that interaction between Pt-CO is weak for Pt/GNS, meaning interaction Pt/GNS is strong. Therefore, CO tolerance of Pt/GNS is higher than Pt/CB. That is possible CO may block strongly Pt for Pt/CB never less on Pt/GNS. The other factor is entropy. We believe that GNS has large surface area. It may be expected to generate high dispersion of Pt. That is why Pt subnano clusters are exist on GNS. Where, it never found on CB.41-43 Figure 7 illustrates it.
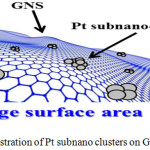 |
Figure 7: Illustration of Pt subnano clusters on GNS.
|
The large surface is required for high dispersion. In case of GNS, high dispersion of small particles Pt are possible dispersed on it. Thereby, Pt subnano clusters tends to strongly binding with GNS compare to CO due to the stability of Pt on GNS. That is because CO tolerance of Pt/GNS will improve. Therefore CO may reduce due to surface oxidation effect.44
Conclusion
Base on the all of data, we conclude that GNS may be expected as a good support material for Pt electrode catalyst on H2-PEMFC. CO tolerance of Pt 30 wt %/GNS catalyst at pH = 1 is higher than Pt 30 wt %/CB (commercial catalyst). That is supposed by two factors namely enthalpy and entropy factors. Pt/GNS catalyst is prospect be an anode catalyst in fuel cells.
References
- Kim, G.; Jhi, S. H. ACS Nano. 2011, 5, 805–810.
CrossRef - Yoo, E. J.; Okada, T.; Akita, T.; Kohyama, M.; Honma, I.; Nakamura, J. Journal of Power Sources. 2011, 196, 110–115.
CrossRef - Zhang, L.; Kim, J.; Chen, H. M.; Nan, F.; Dudeck, K.; Botton, G. A.; Zhang, J.; Liu, R. S. Journal of Power Sources. 2011, 196, 9117–9123.
CrossRef - Rabis, A.; Rodriguez, P.; Schmidt, T. J. ACS Catal. 2012, 2, 864−890.
CrossRef - Joo, J. Y.; Lee, J. K.; Kwon, Y.; Jung, C. R.; Lee, E. S.; Jang, J. H.; Lee, H. J.; Lee, J.; Uhm, S. Fuel Cells. 2010, 10, 926–931.
CrossRef - Nepel, T. C. M.; Lopes, P. P.; Paganin,V. A.; Ticianelli, E. A. Electrochimica Acta. 2013, 88, 217–224.
CrossRef - Shi, G. Y.; Yano, H.; Tryk, D. A.; Watanabe, M.; Iiyama, A.; Uchida, H. Nano Scale, 2016, DOI: 10.1039/c6nr00778c.
CrossRef - Shi, G.; Yano, H.; Tryk, D. A.; Iiyama, A.; Uchida, H. ACS Catal. 2017, 7, 267−274.
CrossRef - Tang, Y.; Yang, Z.; Dai, X. Phys. Chem. Chem. Phys. 2012, DOI: 10.1039/c2cp41441d.
CrossRef - Xu, Y.; Getman, R. B.; Shelton, W. A.; Schneider, W. F. Phys.Chem.Chem.Phys. 2008, 10, 6009–6018.
CrossRef - Wang, D.; Subban, C. V.; Wang, H.; Rus, E.; DiSalvo, F. J.; Abruña, H. D. J. Am. Chem. Soc., 2010, 132, 10218–10220.
CrossRef - Yu, X.; Luo, F.; Yang, Z. RSC Adv., 2016, 6, 98861–98866.
CrossRef - Kwon, K.; Jung, Y.; Ku, H.; Lee, K. H.; Kim, S.; Sohn, J.; Pak, C. Catalysts, 2016, 6, 68, 1–7.
- Yano, H.; Ono, C.; Uchimoto, Y.; Shiroishi, H.; Okada, T.; Saito, M. Chem. Mater. 2006, 18, 4505–4512.
CrossRef - Zhang, L.; Kim, J.; Zhang, J.; Nan, F.; Gauquelin, N.; Botton, G. A.; He, P.; Bashyam, R.; Knights, S. Applied Energy. 2013, 103, 507–513.
CrossRef - Hsieh, Y. C.; Liu, P.; Zhang, Y.; He, P.; Su, D.; Ye, S.; Volkov, V.; Adzic, R. R.; Si, R.; Wang, J. X.; Wu, L.; Zhu, Y.; An, W. Nature Communications. 2013, 4: 2466, 1–9.
- Dong, L.; Tong, X.; Wang, Y.; Guo, X.; Jin, G.; Guo, X. Journal of Solid State Electrochemistry. 2014, 18, 929–934.
CrossRef - Bai, Y. C.; Zhang, W. D.; Chen, C. H.; Zhang, J. Q. Journal of Alloys and Compounds. 2011, 509, 1029–1034.
CrossRef - Li, X.; Qiu, X.; Yuan, H.; Chen, L.; Zhu, W. Journal of Power Sources. 2008, 184, 353–360.
CrossRef - Wang, S.; Jiang, S. P.; White, T. J.; Guo, J.; Wang, X. J. Phys. Chem. C. 2009, 113, 18935–18945.
CrossRef - Reddy, A. L. M.; Ramaprabhu, S. J. Phys. Chem. C. 2007, 111, 16138–16146.
CrossRef - Ruvinskiy, P. S.; Bonnefont, A.; Bayati, M.; Savinova, E. R. Phys. Chem. Chem. Phys. 2010, 12, 15207–15216.
CrossRef - Job, N.; Lambert, S.; Chatenet, M.; Gommes, C. J.; Maillard, F.; Fabry, S. B.; Regalbuto, J. R.; Pirard, J. P. Catalysis Today. 2010, 150, 119–127.
CrossRef - Tang, Y.; Yang, Z.; Dai, X. Phys. Chem. Chem. Phys. 2012, DOI: 10.1039/c2cp41441d.
CrossRef - Kim, G.; Kawazoe, Y.; Lee, K. R. J. Phys. Chem. Lett. 2012, 3, 1989−1996.
CrossRef - Voloshina, E.; Dedkov, Y. Phys. Chem. Chem. Phys. 2012, 14, 13502–13514.
CrossRef - Maeda, K. O.; Morikawa,Y.; Tanaka, S.; Kohyama, M. Surface Science. 2010, 604, 144–154.
CrossRef - Holme, T.; Zhou, Y.; Pasquarelli, R.; O’Hayre, R. Phys.Chem.Chem.Phys. 2010. 12, 9461–9468.
CrossRef - Sun, M.; Lv, Y.; Song, Y.; Wu, H.; Wang, G.; Zhang, H.; Chen, M.; Fu, Q.; Bao, X. Applied Surface Science. 2018, 450, 244–250.
CrossRef - Geng, D.; Hu, Y.; Y.; Li, R.; Li, Sun, X. Electrochemistry Communications. 2012, 22, 65–68.
CrossRef - Wang, H.; Maiyalagan, T.; Wang, X. ACS Catal. 2012, 2, 781−794.
CrossRef - Xin, Y.; Liu, J.; Jie, X.; Liu, W.; Liu, F.; Yin, Y.; Zou, Z.; Gu, J. Electrochimica Acta. 2012, 60, 354–358.
CrossRef - Yoo, E. J.; Nakamura, J.; Okata, T.; Akita, T.; Honma, I.; Kohyama, M. Nano Letters. 2009, 9, 2255–2259.
CrossRef - Marcinek, M.; Song, X.; Kostecki, R. Electrochemistry Communications. 2007. 9, 1739–1743.
CrossRef - Liu, S.; Wang, J.; Zeng, J.; Ou, J.; Li, Z.; Liu, X.; Yang, S. Journal of Power Sources. 2010, 195, 4628–4633.
CrossRef - He, D.; Cheng, K.; Li, H.; Peng, T.; Xu, F.; Mu, S.; Pan, M. Langmuir. 2012, 28, 3979−3986.
CrossRef - Siburian, R.; Kondo, T.; Nakamura, J. The Journal of Physical Chemistry C. 2012, 117, 3635– 3645.
CrossRef - Siburian, R.; Nakamura, J. The Journal of Physical Chemistry C. 2012, 116, 22947–22953.
CrossRef - Iezzi, R. C.; Santos, R. D. M.; Silva, G. C.; Paganin, V. A.; Ticianelli, E. A. J. Braz. Chem. Soc. 2018, 29, 1094–1104.
- Siburian, R. International Research Journal of Pure and Applied Chemistry. 2014, 4, 541–550.
CrossRef - Siburian, R.; Sihotang, H.; Raja, S. L.; Supeno, M.; Simanjuntak, C. Oriental Journal of Chemistry. 2018, 34, 182–187.
CrossRef - Siburian, R.; Sebayang, K.; Supeno, M.; Marpaung, H. Chemistry Select. 2017, 2, 1188–1195.
- Siburian, R.; Sebayang, K.; Supeno, M.; Marpaung, H. Orient. J. Chem. 2017, 33, 134–140.
CrossRef - Modestov, A. D; Tarasevich, M. R.; Filimonov, V. Y.; Davydova, E. S. Electrochimica Acta. 2010, 55, 6073–6080.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









