Utilization of Waste Leaves Biomass of Myrica Esculenta for the Removal of Pb (II), Cd (II) and Zn (II) Ions from Waste Waters
Naveen Chandra Joshi , Ajay Singh and Himanshu Rajput
, Ajay Singh and Himanshu Rajput
Department of Chemistry, Uttaranchal University, Dehradun India.
Corresponding Author E-mail: drnaveen06joshi@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340542
Article Received on : 22-07-2018
Article Accepted on : 20-08-2018
Article Published : 01 Oct 2018
In the present study, we have used the waste leaves of Myrica esculenta for the removal of Pb2+, Cd2+ and Zn2+ ions from the synthetically prepared waste water. The adsorption based removal process has been carried out under the batch system. The batch system was included pH, contact time, dosage, concentration and temperature. The maximum removal efficiency was achieved at optimized conditions i.e. higher contact time, higher pH, lower metal ion concentrations and moderate temperatures. The presence of various organic binding groups was characterized by FTIR spectroscopy. The percentage adsorption of Pb2+, Cd2+ and Zn2+ ions was found 97.02%, 92.52% and 81.99% at pH 6 after contact time 25 minutes. The data of adsorption were tested with Langmuir, Freundlich and Temkin isotherm models. The adsorption capacity of Pb2+, Cd2+ and Zn2+ ions was evaluated as 8.264, 5.617 and 7.751mgg-1 by Langmuir isotherm model.
KEYWORDS:Adsorption; Batch system; Isotherms; Leave waste; Optimized conditions
Download this article as:| Copy the following to cite this article: Joshi N. C, Singh A, Rajput H. Utilization of Waste Leaves Biomass of Myrica Esculenta for the Removal of Pb (II), Cd (II) and Zn (II) Ions from Waste Waters.Orient J Chem 2018;34(5). |
| Copy the following to cite this URL: Joshi N. C, Singh A, Rajput H. Utilization of Waste Leaves Biomass of Myrica Esculenta for the Removal of Pb (II), Cd (II) and Zn (II) Ions from Waste Waters.Orient J Chem 2018;34(5). Available from: http://www.orientjchem.org/?p=49889 |
Introduction
Since from last two or three decades, the exposure of heavy metals in the aquatic bodies has increased very rapidly.1 The presence of heavy metals in the water or waste water causes life threatening problems to all living organisms.2,3 These contaminants are non biodegradable and remain persistent in the environment. The common heavy metals are copper, lead, cadmium, zinc, chromium, nickel, arsenic, iron and cobalt. Biologically, they play an important role in the different physiological activities under the limits. In excess, they are definitely harmful to human and other life forms and some of them are very harmful even at very low concentrations.4-5 The conventional methods used for the removal of heavy metals such as chemical precipitation, electro winning, reverse osmosis, ultrafiltration, ion exchange are suffering from poor performances, less applicability in large scale operations, high cost and generation of harmful chemical sludge. The adsorption process using any local abundant adsorbent is better alternative over conventional methods. It minimizes the use of chemicals, requires very low cost, efficient and can be applicable in large scale waste water treatment methods without any harmful sludge.6,7 This process is depending on the physico-chemical pathways of uptake or as the property of certain biomass to bind and concentrate selected metal ions or other contaminants from aqueous solutions.8-11 Lead (Pb) is a very common heavy metal and released through leaded gasoline, smelting, combustion, battery recycling, lead containing pipes etc. It causes some very harmful effects on reproductive, nervous and urinary systems in the humans. Lead is also a carcinogenic agent and it retards the rate of photosynthesis in plants.12-16 The exposure of cadmium (Cd) in the fresh or saline waters is from the industries like ceramics, metal finishing, alloying, mining etc. It is harmful to human beings and can cause hypertension, renal dysfunction, hepatic injury, lung damage etc.17-23 Zinc is biologically very important in human and other life forms but its extra doses through contaminated water or food causes abdominal pain, dizziness, poor performances of muscles and acute renal failures in human. It releases in water bodies through electroplating, mineral processing, galvanizing plants, paint formulation, porcelain enameling, and vegetable fat producing industries.24,25 The plant Myrica esculenta is abundantly found in the hills of Kumaun and Garhwals, Himanchal Pradesh, Jammu – Kashmir, Sikkim, Arunachal Pradesh, Assam, Meghalaya, Nagaland, Manipur and western Nepal. It is an evergreen gymnospermic plant and has many medicinal properties and is used in the treatment of treating wounds, musculoskeletal disorder and disease of oral cavity. The waste leaves of the plant have been collected from the Kumaun hills of India in the month of June.
Material and Methods
All plastics and borosil reagent bottles were used for preserving adsorbent, stock and working solutions soaked for 12 hrs in 10% nitric acid solution and then washed with deionised water. The chemicals used in the present work were of analytical grade and double distilled water used for washing of glasswares and preparation of all solutions.
Preparation of Adsorbent
The waste leaves of Myrica esculenta were collected in the month of June from the Kumaun hills of Uttarakhand, India. The collected leaves were then washed 3-4 times with deionized water and then dried in the laboratory. The moisture contents of the leaves removed by the drying of leaves in tray dryer between the temperature 60-650C under controlled conditions. After that, these leaves have been grinded and sieved into the particle size 0.125 mm (BSS120). The prepared leaf powder then preserved in sealed bottles and 0.1 g was used for FTIR studies.
Preparation of waste water and adsorption study:
The waste water containing Pb2+, Cd2+ and Zn2+ ions was prepared by dissolving the salts lead nitrated Pb (NO3)2 , cadmium nitrate Cd (NO3)2 and zinc sulphate Zn (SO4)2.7H2O in double distilled water, separately. For making 1000 mgl-1 stock solutions, 1.598 g Pb (NO3)2, 2.103 g Cd (NO3)2 and 4.397 g Zn (SO4)2.7H2O were dissolved separately in deionized waters. The pH of stock solutions was adjusted 4 by digital pH meter (Model: Teshcon Sr No 15J520). The pH of all stock and working solutions was adjusted by using 0.1 M HCl or 0.1 M NaOH solutions. A required amount of adsorbent (0.1 to 1g) was treated with 100 ml of desired working solutions containing 10 to 50 mg/L of Pb2+, Cd2+ and Zn2+ ions in a 250 ml of Erlenmeyer flask at constant rpm 170 and pH 1 to 6. The contact times 10 to 70 minutes and temperatures 10 to 700C have been used in the overall removal process. After completion the reaction, the solutions were filtered and metal ion concentrations measured in filtrate using Atomic Absorption Spectroscopy (Thermo Scientific: iCE 3000 Series). The percentage adsorption of metal ios onto the adsorbent is calculated by following formula:
![]()
Where, Ci and Cf are initial and final concentration of metal ions in the aqueous solution.
 |
Figure 1: (A) Myrica esculenta (B) Collected waste leaves Click here to View figure |
Results and Discussion
FTIR Characterizations
Fourier transform infrared spectroscopy (FTIR) is depending on the interference of the electromagnetic radiation and the absorption spectra obtained by the techniques of interference. The FTIR spectra explain the presence of organic binding groups that are responsible for the interactions with metal ions. The FTIR spectra of adsorbent prepared from the waste leave biomass of Myrica esculenta in the range of 4000-400 cm-1 are represented in Fig.2. The sharp peaks at 3425 cm-1, 3401 cm-1, 3371 cm-1, 2923 cm-1, 2853 cm-1, 1621 cm-1, 1541 cm-1, 1368 cm-1 and 1231 cm-1 have been found due to presence of different organic groups. The sharp adsorption bands at 3425 cm-1, 3401 cm-1, 3371 cm-1, 2923 cm-1 and 2853 cm-1 are indicating that the presence of –OH, –NH, and C–H (aliphatic) groups on the surface of adsorbent. The peaks 1621 cm-1, 1541 cm-1, 1368 cm-1, 1231 cm-1 are assigned to C=O, C=C, C–N, C-O, C-C etc on the surface of unloaded leaf powder.26-29
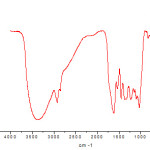 |
Figure 2: FTIR Spectra for the adsorbent prepared from the waste leaves of Myrica esculenta Click here to View figure |
Effect of pH and contact time
The pH of solution is very important parameter in the adsorption phenomenon. At pH 3, the removal efficiencies of lead, cadmium and zinc has been recorded 10.79, 10.11 and 26.75% and increases to 26.79, 14.71 and 48.03% at pH 4. The maximum adsorption was found at pH 6; that has been found to be 97.02, 92.52 and 81.99% for lead, cadmium and zinc. It is due to the factor that all the binding organic groups undergo protolysis and become positively charged at lower pH. It is therefore, repulsion occurs between metal ions and binding groups that result poor adsorption30-32 (Fig 3). A minimum time is required for the interactions of metal ions and adsorbent. The percentage adsorption was found to be 29.88, 13.97 and 52.81% for Pb (II), Cd (II) and Zn (II) at contact time 30 minutes and after 60 minutes it increases to 47.65, 39.88 and 68.90% at pH 4 and 10 mg/L concentration of metal ions (Fig 4). After that, the adsorption becomes constant due to the occupation of all the active sites by the metal ions.24,25
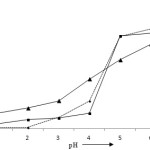 |
Figure 3: Effect of pH on the adsorption Pb (II), Cd (II) and Zn (II) ions. Click here to View figure |
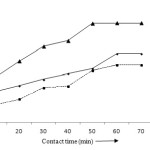 |
Figure 4: Effect of contact time on the adsorption of Pb (II), Cd (II) and Zn (II) ions. Click here to View figure |
Effect of dosage and concentration
The removal efficiency of metal ions is usually increased with the amount of adsorbents. Initially, the adsorption of lead, cadmium and zinc was found to be 4.48, 1.39 and 12.48% and that recorded 10.79, 8.79 and 20.81% at the dosage 0.4 g. The highest removal was achieved at 1 g after 25 minutes. At this amount, 31.08% lead, 18.99% cadmium and 5.601% zinc have been removed at pH 4 (Fig 5). In general, the number of active sites increases with the increase of the amount of adsorbent and that results maximum adsorption.33 The experimental data of adsorption of all these metal ions indicate that the removal efficiency decreases with the increase in the concentrations of solution but the amount of adsorbate metal ions (mg/g) increases. This can be explained as the generation of motive force of concentration gradient as the initial metal ion concentration.34,35 At initial metal ion concentration (10 mg/L), the removal efficiency for Pb (II), Cd (II) and Zn (II) ions was found to be 27.09, 15.69 and 40.75% and that decreases to 11.75, 7.92 and 13.99% (Fig 6).
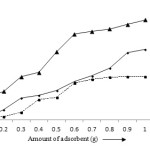 |
Figure 5: Effect of dosage on the adsorption of Pb (II), Cd (II) and Zn (II) ions. Click here to View figure |
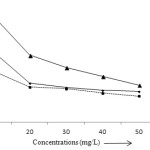 |
Figure 6: Effect of concentration on the adsorption of Pb (II), Cd (II) and Zn (II) ions. Click here to View figure |
Effect of temperature
The adsorption of metal ions increases with increase in temperature of the aqueous solutions due to the escaping tendency of adsorbate metal ions to the entire space of the surface of adsorbents. After certain temperature, the adsorption becomes constant or decreases. It may be attributed to the dissolution of adsorbed metal ions in the solutions.6 At initial temperature 10°C, the removal efficiency of Pb (II), Cd (II) and Zn (II) ions was recorded 25.99, 10.25 and 44.41%. It increases very smoothly to 32.90, 34.03 and 67.91% at 60°C. After 60°C, the adsorption of lead, cadmium and zinc becomes about constant (Fig 7).
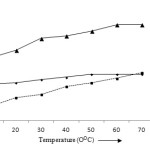 |
Figure 7: Effect of temperature on the adsorption of Pb (II), Cd (II) and Zn (II) ions |
Adsorption isotherms
In general, the adsorption isotherms explain the distribution of adsorbate metal ions in between two phases i.e. solid and liquid and existence of an equilibrium state. Suitable designed isotherms indicate the type of adsorption and critical optimization of the utilization of adsorbents. Langmuir isotherm model explains the formation of monolayer of metal ions on the surface of adsorbents that contain a finite number of binding groups. These binding groups are arranged in a regular pattern.36,37 Mathematically, the Langmuir isotherm model can be represented as below:
Cf /Qe = 1/Klb + Cf/Kl
Where, Cf and Qe are the equilibrium concentrations (mgl-1) of metal ions in aqueous solution and their amount adsorbed on the surface of adsorbent (mgg-1). The Langmuir constant Kl and b represents adsorption capacity (mgg-1) and adsorption equilibrium constant (Lmg-1). The values of Kl and b have been evaluated from the graph Cf / Qe vs Cf (Fig 8). For Pb (II), Cd (II) and Zn (II), the values of Kl and b are found 8.264, 5.617 and 7.751mgg-1 and 1.001, 1.002 and 1.013 Lmg-1. The values of regression (R2) indicates that zinc (R2 = 0.977) follows Langmuir model more than cadmium (R2 = 0.961) and lead (R2 = 0.837). The dimensionless factor RL of Langmuir model is represented as below:
RL=1/1+bCi
Where, b and Ci are the Langmuir constant and the initial metal ion concentration (mgl-1). The value of RL determines adsorption i.e. favorable (0<RL<1), unfavorable (RL>1), linear (RL=1) or irreversible (RL=0). Here the value of RL was found to be <1 in all cases of applied batch system of concentration dependency. The Freundlich isotherm model is explained as the presence of different binding groups that involved in the interaction with metal ions varying with different energies on the heterogeneous surface of the adsorbents.38 The Freundlich isotherm model is mathematically given as below:
log Qe = log Kf + 1/n log Cf
Where, Qe and Cf are the amount of metal ion adsorbate and final concentration of aqueous solution in mgg-1 and mgl-1. The constants Kf and 1/n are the adsorption capacity and intensity of adsorption and their values are calculated from the graph log Qe vs log Cf (Fig 9). The values of Kf for lead, cadmium and zinc are evaluated as 2.691, 3.176 and 1.927 mgg-1, respectively. From the values of R2, we can arrange all the metal ions for the suitability for Freundlich isotherm model as Cd (II) = Zn (II) > Pb (II) (Table 1).
Temkin isotherm model is generally used for the interactions between adsorbed metal ions and a linear decrease of heat of adsorption for the coverage due to metal ions on the surface of adsorbents.6 The Temkin isotherm model is mathematically defined as:
Qe = X + Y ln Cf
Where Cf is the equilibrium concentration of metal ions in mgl-1, Qe is the amount of adsorbate metal ions in mgg-1, X and Y are adsorption capacity and Temkin constant (Table 1).
Table 1: Isotherm parameters for the adsorption of Pb (II), Cd (II) and Zn (II) ions.
|
Isotherm models |
Parameters |
Calculated values |
|
Langmuir isotherm model |
Kl (mg/g) |
11.494 |
|
b (L/mg) |
0.0318 |
|
|
R2 |
0.920 |
|
|
Freundlich isotherm model |
Kf (mg/g) |
0.769 |
|
1/n |
0.574 |
|
|
R2 |
0.989 |
|
|
Temkin isotherm model |
X (mg/g) |
2.775 |
|
Y |
2.475 |
|
|
R2 |
0.940 |
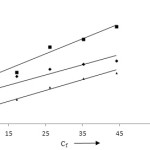 |
Figure 8: Langmuir isotherm model. |
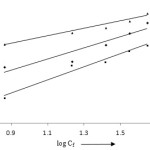 |
Figure 9: Freundlich isotherm model |
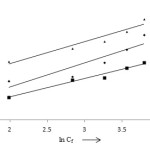 |
Figure 10: Temkin isotherm model |
Conclusions
The experimental data of adsorption shows that the waste leaves of Myrica esculenta are potential adsorbents for the removal of Pb (II), Cd (II) and Zn (II) ions from the waste waters. The maximum adsorption of metal ions was observed at contact time 60 minutes, pH 6, dosage 1 g and temperature 600C. The equilibrium data of adsorption have been tested with Langmuir, Freundlich and Temkin isotherm models. The regression values and other evaluated constants indicate the favorable adsorption found in the applying batch conditions. It was observed that the adsorption of Pb (II), Cd (II) and Zn (II) ions on the surface of leaf powder prepared from Myrica esculenta Freundlich isotherm is more favorable than Langmuir and Temkin isotherm model.
Acknowledgement
We are thankful to the Department of Chemistry, Uttaranchal University Dehradun (India) for providing all necessary facilities during overall research work.
References
- Filote, C.; Ungureanu, G.; Boaventura, R.; Santos, S.; Volf I. Process Safety and Environmental Protec. 2017, 108, 34-43
CrossRef - Mohammed, A.S.; Kapri, A.; Goel, R. Environmental Pollution, Springer, Dordrecht, Netherlands 2011, 20, 1-28
- Jayakumar ,V.; Govindaradjane, S. J. Advanced Chemical Sci. 2017, 3, 480-484
- Martins, R.J.E.; Pardo, R.; Boaventura, R.A.R., Water Res. 2004, 38, 693–699
CrossRef - Uluozlu, O.D.; Sari, A.; Tuzen, M.; Soylak, M. Bioresour. Technol. 2008,99, 2972–3298
CrossRef - Joshi, N.C.; Bahuguna, V. Rasayan J. Chem. 2018, 11, 142-150
- Joshi, N.C. Res.J. Life Bio. Pharm. Chem. Sci. 2018, 4,59
- Fourest, E.; Roux, J.C. Appl. Microbiol. Biotechnol. 1992, 37,399-403
CrossRef - Petersen, F.; Aldrich, C; Esau, A.; Qi, B.C. WRC Report 2005, 1, 100
- Volesky, B. Water Res. 2007, 41, 4017-4029
CrossRef - Ahalya, N; Ramchandra, T.V.; Kanamadi, R.D. Research J. of Chemistry and Environ. 2003, 7, 4
- Nikolas, C; Papanikolaou, G; Eleftheria; Hatzidaki; Belivanis, S; George, N; Tsatsakis, T.A.M. Med. Sci. Monit. 2005, 11, 329-336
- Wani, A.L.; Anjum, A.R.A.; Usmani, J.A. Interdiscip. Toxicol. 2005, 8, 55–64
CrossRef - Iram, S; Shabbir, R.; Zafar, H; Javaid, M. Arabian J. Sci. and Eng. 2015, 40, 1867–1873
CrossRef - Saletnik, B.; Zaguła, G.; Grabek-Lejko, D. Environ. Earth Sci. 2017, 76, 574
CrossRef - Farhan, S.N.; Khadom, A.A. Int. J. Ind. Chem. 2015, 6, 119
CrossRef - Ahmedna, M.; Marshall, W.E.; Husseiny, A.A.; Rao, R.M.; Goktepe, I. Water Res. 2004, 38, 1062–1068
CrossRef - Volesky, B. Biosorption of Heavy Metals, CRC Press, Boca Raton, 1990
- Lo, W.; Chua, H.; Lam, K.H.; Bi, S.P. Chemosphere 1999, 39, 2723–2736
CrossRef - Davis, T.A.; Volesky, B.; Vieira, R.H.S.F. Water Res. 2000, 34, 4270–4278
CrossRef - Hajialigol, S.; Taher, M.A.; Malekpour, A. Ads. Sci. Technol. 2006, 24, 487–496
CrossRef - Tang, Q.; Zhou, T.; Gu, F.; Wang, Y.; Chu, J.M. J. Cent. South Uni. 2017, 24, 1941
CrossRef - Cao, Y.; Zhang, S.; Wang, G.; Xu, X. Environ. Sci. Pollut. Res. 2017, 24, 8525
CrossRef - Bhandari, N.S.; Joshi N.C.; Kumar, S. Environ Sci: An Ind J. 2011, 6,75-79
- Joshi N.C.; Bhandari, N.S.; Kumar, S. Environ Sci: An Ind J. 2011, 6, 148-153
- Elangovam, R.; Philip, L.; Chandraraj, K. J. Hazard. Mater. 2008, 152,100-112
CrossRef - Wang, F.; Pan, Y.; Cai, P.; Guo, T.; Xio, H. Biores. Tech. 2017, 241, 482-490
CrossRef - Ozacar, M.I.; Ayhan Sangil, H.; Turkmenler Chem. Eng. J. 2008, 143, 32-42
- Iqbala, M.; Saeeda, A.; Iqbal Zafar, S. J. Hazard. Mater. 2009, 164, 161-171
CrossRef - Romera, E.; Gonzalez, F.; Ballester, A.; Blazquez Munoz, J.A. J. Bioresour. Tech. 2007, 98,3344–3353
CrossRef - Ibrahim, W.M.; Aziz, Y.S.A.; Hamdy, S.M.; Gad, N.S. J. Biorem. and Biodeg. 2018, 9, 1
- Bilal, M.; Rasheed, T.; Raza, A.; Nadeel, F.; Iqbal, H.M.N. Mar. Drugs 2018, 16, 65
CrossRef - Ramzy, B.; Nessim; Ahmad, R; Bassiouny; Hermine, R; Zaki; Madelyn; N.; Moawad; Kamal; M.; Kandeel,Chemistry and Ecology 2011, 27, 579-594
- Al-Fakih, A.A.; Gharieb, M.M.; Ali, M.I. Al-Jazeera Univ. J. 2018, 1, 5-29
- Puranik, P.R.; Paknikar, K.M. Biotechnol. Progr. 1999, 15, 228–237
CrossRef - Langmuir, I. J. Am. Chem. Soc. 1998, 40, 1361–1403
CrossRef - Aziam, R.; Chiban, M.; Eddaoudi, H.; Shoudani, A.; Zerbet, M.; Sinan, F. Eur. Phys. J. Spec. Top. 2017, 226, 977
CrossRef - Ho, Y.S.; Porter, J.F.; McKay, G. Water, Air & Soil Pollution 2002, 141, 1.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









