HPLC Analysis of Chemical Composition of Selected Jordanian Medicinal Plants and Their Bioactive Properties
Fuad Al-Rimawi1 , Fadi Alakhras2
, Fadi Alakhras2 , Wael A. Al-Zereini3
, Wael A. Al-Zereini3 , Hammad K. Aldal'in4
, Hammad K. Aldal'in4 , Saleh Abu-Lafi5, Ghassab M. Al-Mazaideh6
, Saleh Abu-Lafi5, Ghassab M. Al-Mazaideh6 and Haya J. Ayyal Salman3
and Haya J. Ayyal Salman3
1Department of Chemistry, Faculty of Science and Technology, Al-Quds University, P.O. Box 20002, Jerusalem, Palestine.
2Department of Chemistry, College of Science, Imam Abdulrahman Bin Faisal University, Dammam 31441, Saudi Arabia.
3Department of Biological Sciences, Faculty of Science, Mutah University, P.O. Box 7, Mutah 61710, Al-Karak, Jordan.
4Department of Medical Support, Al-Balqa Applied University, Al-Karak University College, Al-Karak, Jordan.
5Faculty of Pharmacy, Al-Quds University, P.O. Box 20002, Jerusalem, Palestine.
6Department of Chemistry and Chemical Technology, Faculty of Science, Tafila Technical University, P.O. Box 179, Tafila 66110, Jordan.
Corresponding Author E-mail: falrimawi@staff.alquds.edu
DOI : http://dx.doi.org/10.13005/ojc/340522
Article Received on : 18-08-2018
Article Accepted on : 30-09-2018
Article Published : 17 Oct 2018
Three medicinal plants grown wild in Jordan, namely Achillea santolina L, Achillea fragrantisimma, Asteriscus graveolens (Forssk) Less, were extracted with ethyl acetate by continuous shaking at room temperature for three days. The antibacterial activity of the crude extract was evaluated. The extracts were analyzed for their phenolic and flavonoids content by HPLC-PDA. The HPLC analysis of the plant extracts revealed the presence of flavonoids and phenolic compounds in the three plant extracts. Results revealed a strong antibacterial activity of A. graveolens against three bacterial strains (B. subtilis, E. coli, and S.aureus) while A. fragrantissima inhibited the growth of B. subtilis. Bioactivities were attributed mainly to the immense content of phenol-based compounds in plants.
KEYWORDS:Achillea Santolina L, Achillea Fragrantisimma; Asteriscus Graveolens (Forssk.) Less; Antibacterial Activity; Medicinal Plants
Download this article as:| Copy the following to cite this article: Al-Rimawi F, Alakhras F, Al-Zereini W. A, Aldal'in H. K, Abu-Lafi S, Al-Mazaideh G. M, Salman H. J. A. HPLC Analysis of Chemical Composition of Selected Jordanian Medicinal Plants and Their Bioactive Properties. Orient J Chem 2018;34(5). |
| Copy the following to cite this URL: Al-Rimawi F, Alakhras F, Al-Zereini W. A, Aldal'in H. K, Abu-Lafi S, Al-Mazaideh G. M, Salman H. J. A. HPLC Analysis of Chemical Composition of Selected Jordanian Medicinal Plants and Their Bioactive Properties. Orient J Chem 2018;34(5). Available from: http://www.orientjchem.org/?p=51002 |
Introduction
Huge developments in the pharmaceutical industries and chemistry are taking place nowadays, due to new findings in herbal medicine. Plants were used as the important sources of most drugs for eliminating pain and treating diseases.1 In fact, using naturally derived bioactive products had led to overcome the side effects and new resistant pathogens of the most known synthetic drugs. As many of natural products haven’t been identified until now, it is important to find new bioactive compounds that can be used for treating various ailments. Plants have been considered as natural sources of potentially safe biologically active metabolites.2,3 The variety of secondary metabolites in plants could be used as pure secondary metabolites or in a synergy form to have good bioactivities in several biological systems.4 Actually, modern medicine depends on numerous therapeutic agents that were isolated from medicinal plants such as Colchicines, digitoxin, quinine, aspirin, taxol, morphine and atropine.5,6
In Jordan, there are a total of 2500 plant species, belonging to 700 genera; 485 species from 99 different plant families are used as medicinal plants in traditional medicine.7-9 However, usage of these plant as folk medicine without any awareness of their side effects or dose toxicity, impose drawback in their medicinal interest.10 Moreover, many studies were carried out on Jordanian folk medicinal plants, mainly on their bioactivity such as antimicrobial and antioxidant activities11-13; in vitro screening of phytochemistry14,15 and ethnobotany.16,17,6 Hence, this study aimed to evaluate the bioactivity and to analyze the chemical compositions by HPLC-PDA of three selected Jordanian medicinal plants that are used as a folk medicine in treating various diseases (Table 1).
Table 1: List of selected Jordanian medicinal plants and their usage.
| Scientific name | Traditional use/medical use | Reference |
| Achillea santolina L.(Labiatae) | Carminative, depurative, stomachaches, antispasmodic and anti-diabetes, | 13 |
| Achillea fragrantisimma(Labiatae) | Carminative, depurative, stomachaches, antispasmodic, anti-diabetes, anti-tumor and rheumatic pain | 2 |
| Asteriscus graveolens (Forssk.) Less.(Asteraceae) | Anti-diabetic, anti-inflammatory, for gastric and bowel diseases, diuretic, hypotensive and depurative | 18 |
Experimental
Plant Materials Collection and Extraction
All plant samples were collected from different localities in the southern part of Al-Karak governorate-Jordan. They were collected on the basis of traditional practices by herbalists and healers and were identified according to Al-Eisawi19 and taxonomically authenticated by Dr. Ferryal Al-khreisat, Biology Department, Mutah University.
Plants aerial parts were dried in shade until constant weight and pulverized. 100 g of powdered plant materials were soaked in 1L of ethyl acetate with continuous shaking (150 rpm, Forma Orbital Shaker, Thermo electron cooperation, USA) at room temperature for 3 days. The filtrates were concentrated in vacuo at 45°C using rotary evaporator (Buchi R-215, Switzerland) and the resulting residue was dissolved in methanol to a final concentration of 0.1 g/ml.
In Vitro Antibacterial Activity Determination
The antibacterial activity of plants crude extracts was determined by agar diffusion test and the minimum inhibitory concentration (MIC) was determined by serial dilution assay according to the Clinical and Laboratory standards Institute guidelines.20 Staphylococcus aureus ATCC 43300, Escherichia coli ATCC 25922, Micrococcus luteus ATCC 10240,and Bacillus subtilis ATCC 6633, seeded on LB agar plates (0.5% tryptone, 0.5% yeast extract, 1% NaCl, 1.8% agar) were used as a test bacterial strains.
In disk diffusion assay 0.5, 1 and 1.5 mg/disc of plant crude extract were used, while 2 mg/ml of the plant extract and10µg/ml positive control (penicillin G) were used as initial concentrations in serial dilution assay.
HPLC System and Chromatographic Conditions
The HPLC is a Waters Alliance (e2695 separations module), equipped with 2998 Photo diode Array detector (PDA). Data acquisition and control were carried out using Empower 3 chromatography data software (Waters, Germany).
The HPLC analytical experiments of the crude extracts of the three aerial samples were run on ODS column of Waters (X Bridge, 4.6 ID x 150 mm, 5 μm) with guard column of Xbridge ODS, 20 mm x 4.6mm ID, 5 μm. The mobile phase is a mixture of acetic acid in water (0.5%) (Solvent A) and acetonitrile (solvent B) ran in a linear gradient mode. 100% (solvent A) descended to 70% (solvent A) in 40 minutes. Then to 40% (solvent A) in 20 minutes and finally to 10% (solvent A) in 2 minutes and stayed there for 6 minutes and then back to the initial conditions in 2 minutes. The HPLC system was equilibrated for 7 minutes with the initial acidic water mobile phase (solvent A) before injecting next sample. All the samples were filtered with a 0.45 mm PTFE filter. The PDA wavelengths range was from 210-500 nm. The flow rate was 1 ml/min. Injection volume was 20 ml and the column temperature was set at 25°C.
Results and Discussions
HPLC-PDA Profiles of the Extracts
Figure 1 shows the chromatogram of the crude extract of Achillea santolina L at 340 nm. This wavelength was selected because the major 6 peaks showed a maximum absorption at this wavelength. The eluted compounds were detected in the range of 47-58 minutes; indicating nonpolar compounds. At 258 nm, a new major peak eluted at 42.8 minutes was appeared as well as another 3 minor peaks at 28.0, 41.1, and 42.1 minutes (Figure 2). The eluted compounds were detected in the range of 28-57 minutes; indicating polar and nonpolar compounds combination. The UV-Vis ranges of these compounds were in the range of 210-350nm indicating flavonoids abundance.21 These compounds are not part of the standards injected as per their retention and UV-Vis spectra tell.
As shown in figure 2, about 10 minor compounds were seen, of which only one compound showed major dominance indicating flavonoids abundance.21
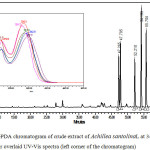 |
Figure 1: HPLC-PDA chromatogram of crude extract of Achillea santolinaL at 340 nm, their overlaid UV-Vis spectra (left corner of the chromatogram). Click here to View figure |
 |
Figure 2: HPLC-PDA chromatogram of crude extract of Achillea santolinaL at 258 nm, their overlaid UV-Vis spectra (right corner of the chromatogram), and the UV-Vis spectrum of the major peak eluted at 42.8 minutes (left side to the major peak). Click here to View figure |
Figure 3 shows the chromatogram of the crude extract of Achillea fragrantisimma at 350 nm with main peak eluted at 44.8 minutes and other 11 minor compounds showed a maximum absorption at this wavelength. The eluted compounds were detected in the range of 21-49 minutes; indicating polar and nonpolar compounds combination. The UV-Vis ranges of these compounds were in the range of 210-350 nm.
 |
Figure 3: HPLC-PDA chromatogram of crude extract of Achillea fragrantisimma at 350 nm, their overlaid UV-Vis spectra (left corner of the chromatogram). |
Figure 4 shows the chromatogram of the crude extract of Asteriscus graveolens (Forssk) Less (Asteraceae) at 350 nm with two main peaks (eluted at 33.4 and 38.9 minutes) and other six peaks showed a maximum absorption at this wavelength. The eluted compounds were detected in the range of 14-55 minutes; indicating polar and nonpolar compounds combination. The UV-Vis ranges of these compounds were in the range of 210-350 nm.
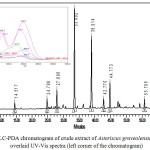 |
Figure 4: HPLC-PDA chromatogram of crude extract of Asteriscus graveolensat 350 nm, their overlaid UV-Vis spectra (left corner of the chromatogram). |
Antibacterial Activity of the Plant Extracts
The tested crude extracts, except that of A. santolina showed weak to moderate antibacterial activity against the Gram positive B. subtilis (Table 2). The inhibition zones caused by applied extracts were 10-15 mm at 1.5 mg/disc. Interestingly, A. graveolens inhibited the growth of all tested bacterial strains with MIC 0.5-1 mg/ml. Meanwhile; A. fragrantissima inhibited the growth of B. subtilis at MIC 0.25 mg/ml (Table 3).
Numerous studies described the various pharmacological effects of Achillea extracts in management of several diseases. Among its species, A. fragrantissima and A. santolina were reported to contain metabolites with various bioactivities including antibacterial potentials.22 These activates were attributed to broad ranges of secondary metabolites such as flavonoids, phenolic acids, cumarines, terpenoids and sterols.
In accordance to our finding, the antibacterial effect of hydro-alcoholic and aqueous extracts of A. fragrantissma was reported against Gram positive bacteria with pronounced resistance among Gram negative ones23; however, its phenolic extract showed weak effect against Pseudomonas mirabilis with MIC 25 mg/ml.24 Moreover, its essential oil extract exerted a bactericidal effect on both Gram positive and negative bacteria as well as on the yeast Candida albicans.25,26
Although A. santolina crude extract was devoid of antibacterial potential, however, it was reported previously that it was active against some species of Gram positive and negative bacteria with MIC 40-60 mg/ml27 indicating that it might be active at concentrations at least 40 fold than that applied in this study. Furthermore, the activity of A. graveolens against both Gram positive and negative tested bacterial strains reported herein coincided with documented activity of the same plant species collected from Algeria.28 Therein, the ethyl acetate crude extract of A. graveolens revealed weak to moderate activity against Gram positive bacterial and was inactive to Gram negative bacteria with MIC 312-625 µg/ml and 1 mg/ml, respectively.
Chemical analysis of ethyl acetate crude extracts of these three plants revealed presence of phenolic compounds and flavonoids in addition to mixtures of nonpolar and polar metabolites. Presence of these metabolites in association of tannins and terpenoids confer antimicrobial and antioxidant activities for such extracts. Intriguingly, flavonoids are able to form complexes with biological molecules and microbial cell wall and may deteriorate their role, thereby causing microbial growth inhibition.29,22,28
Table 2: Antibacterial activity of plants crude extract against susceptible bacterial strains in agar diffusion test.
| Plant name | Inhibition zone (mm ± SDa) 1/1.5 (mg/disc) | ||
| B. subtilis | E. coli | S. aureus | |
| A. santolina | NAb | NA | NA |
| A. fragrantissima | 13.7 ± 1.5/14.7 ± 0.6 | NA | NA |
| A. graveolens | 8.7 ± 1.2/ 10.7 ± 0.6 | 7.7 ± 0.6/ 11.7 ± 0.6 | 9.3 ± 0.6/ 11.3 ± 1.2 |
a) Standard deviation.
b) NA: Inactive n.
Table 3: Minimal inhibitory concentration of plants crude extract against susceptible bacteria.
| Plant name | MIC (mg/ml) | ||
| B. subtilis | E. coli | S. aureus | |
| A. fragrantissima | 0.25c | ˃ 2 | ˃ 2 |
| A. graveolens | 0.5s | 1s | 0.5c |
| Penicillin G | 0.00016c | ˃ 0.01 | 0.00032c |
c: bacteriocidal / s: bacteriostatic
Conclusion
The extract from A. graveolens exhibited a significant in-vitro antibacterial activity of all tested bacterial strains with MIC 0.5-1 mg/ml that can be an important source of natural antibacterial. This bioactivity is quite significant and could present alternative treatments for many infections. The extracts of the three plants are rich with phenolic and flavonoids.
Conflict of Interest
There is no conflict of interest.
Acknowledgments
Authors wish to acknowledge Mutah University and Al-Quds University for providing facilities, and encouragement.
References
- Saad, B.; Azaizeh. H.; Said. O. Tradition and perspectives of Arab herbal medicine: a review. Evid Based Complement Alternat Med. 2005, 2, 475-9.
CrossRef - Hassawi, D.; Kharma, A. Antimicrobial activity of some medicinal plants against Candida albicans. J. Biol. Sci. 2006, 6, 109-114.
CrossRef - Nenaah G. Antibacterial and antifungal activities of (beta)-carboline alkaloids of Peganum harmala (L) seeds and their combination effects. Fitoterapia. 2010, 81, 79-782.
CrossRef - Cioch, M.; Satora, P.; Skotniczny, M.; Semik-Szczurak, D.; Tarko, T. Characterisation of Antimicrobial Properties of Extracts of Selected Medicinal Plants. Pol. J. Microbial. 2017, 66, 463-472.
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431-441.
CrossRef - Hudaib, M.; Mohammad, M.; Bustanji, Y.; Tayyem, R.; Yousef, M.; Abuirjei, M.; Aburjai, T. Ethnopharmacological survey of medicinal plants in Jordan, Mujib Nature Reserve and surrounding area. J. Ethnopharmacol. 2008, 120, 63-71.
CrossRef - Oran, S.A.; Al-Eisawi, D.M. Checklist of medicinal plants in Jordan. Dirasat. 1998, 25, 84-112.
- Afifi, FU. ; Abu-Irmaileh, B. Herbal medicine in Jordan with special emphasis on less commonly used medicinal herbs. J. Ethnopharmacol. 2000, 72, 101-110.
CrossRef - Al-Momani, W.; Abu-Basha, E.; Janakat, S.; Nicholas, R.; Ayling, R. In vitro antimycoplasmal activity of six Jordanian medicinal plants against three Mycoplasma species. Trop Anim Health Prod. 2007, 39, 515–519
CrossRef - Al-Mazaideh, G. M. Carbohydrates as Green Corrosion Inhibitors of Cooper: Ab initio study. Jordan J. Chem. 2017, 12, 189-200.
- Aburjai, T.; Darwish, R.M.; Al-Khalil, S.; Mahafzah, A.; Al-Abbadi A. Screening of antibiotic resistant inhibitors from local plant materials against two different strains of Pseudomonas aeruginosa. J. Ethnopharmacol. 2001, 76, 39-44.
CrossRef - Tawaha, K.; Alali, F.Q.; Gharaibeh, M.; Mohammad, M.; El-Elimat, T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007, 104, 1372-1378.
CrossRef - Darwish, R.M.; Aburjai, T. Antimicrobial activity of some medicinal plants against different Candida species. Jordan J. Pharm. Sci. 2011, 4, 70-80
- Flamini, G.; Cioni, P.L.; Morelli, I.; Bader, A. Essential oils of the aerial parts of three Salvia species from Jordan: Salvia lanigera, S. spinosa and S. syriaca. Food Chem. 2007, 100, 732-735.
CrossRef - Abu-Darwish, M.; Al-Ramamneh, E.; Salamon, I.; Abu-Dieyeh, Z.; Al Nawaiseh, M. Al-Bdour, T. Determination of essential oil bioactive components and rosmarinic acid of Salvia officinalis cultivated under different intra-row spacing. Not. Sci. Bio. 2013, 5, 198-203.
CrossRef - Abu-Irmaileh, B.E.; Afifi, F. Herbal medicine in Jordan with special emphasis on commonly used herbs. J. Ethnopharmacol. 2003, 89, 193-197.
CrossRef - Aburjai, T.; Hudaib, M.; Tayyema, R.; Yousef, M.; Qishawi, M. Ethnopharmacological survey of medicinal herbs in Jordan, the Ajloun Heights region. J. Ethnopharmacol. 2007, 110, 294-304.
CrossRef - Ziani, B.E.C.; Calhelha B, C.; Barreira, J.; Barros B, L.; Hazzit A, M.; Ferreira, I. C.F.R. Bioactive properties of medicinal plants from the Algerian flora: selecting the species with the highest potential in view of application purposes. Ind. Crop. Prod. 2015, 77, 582-589.
CrossRef - Al-Eisawi, D.M. Field Guide to Wild Flowers of Jordan and Neighboring Countries. Press— Foundation Al-Rai, Jordan. 1998.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; Twenty-second informational supplement (M100-S22). Wayne, PA: CLSI. 2012
- Al-Rimawi, F.; Abu-Lafi, S.; Abbadi. J.; Alamarneh, A.; Sawahreh, R.; Odeh, I. Analysis of phenolic and flavonoids of wild Fphedra alata plant extracts by LC/PDA and LC/MS and their antioxidant activity. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 130-141
CrossRef - Tabanca, N.; Demirci, B.; Gurbuez, I.; Demirci, F.; Becnel, J. J.; Wedge, D. E.; Baser, K. H. Essential oil composition of five collections of Achillea biebersteinii from central Turkey and their antifungal and insecticidal activity. Nat. Prod. Commun. 2011, 6, 701-706
- Hammad, H. M.; Matar, S. A.; Litescu, S. C.; Abuhamdah, S.; Al-Jaber, H. I.; Afifi, F. U. Biological activities of the hydro-alcoholic and aqueous extracts of Achillea fragrantissima (Forssk.) grown in Jordan. Nat. Sci. 2014, 6, 23-30
- Aqel, H.; Al-Charchafchi, F.; Ghazzawi, D. Biochemical, antibacterial and antifungal activity of extracts from Achillea fragrantissima and evaluation of volatile oil composition. Pharm. Sinica. 2012, 3, 349-356
- El-Shazly, A. M.; Hafez, S. S.; Wink, M. Comparative study of the essential oils and extracts of Achillea fragrantissima (Forssk.) Sch. Bip. and Achillea santolina L.(Asteraceae) from Egypt. Pharmazie. 2004, 59, 226-230
- Aburjai, T.; Hudaib, M. Antiplatelet, antibacterial and antifungal activities of Achillea falcata extracts and evaluation of volatile oil composition. Pharmacog. Mag. 2006, 2, 191-198
- Khalil, A.; Dababneh, B. F.; Al-Gabbiesh, A. H. Antimicrobial activity against pathogenic microorganisms by extracts from herbal Jordanian plants. J. Food Agric. Env. 2009, 7, 103-106
- Ramdane, F.; Essid, R.; Mkadmini, K.; Hammami, M.; Fares, N.; Mahammed, M. H.; El Ouassis, D.; Tabbene, O.; Limam, F.; Hadj, M. D. O. Phytochemical composition and biological activities of Asteriscus graveolens (Forssk) extracts. Process Biochem. 2017, 56, 186-192
CrossRef - Kamatou, G. P. P.; Makunga, N. P.; Ramogola, W. P. N.; Viljoen, A. M. South African Salvia species: a review of biological activities and phytochemistry. J. ethnopharmacol. 2008, 119, 664-672.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









