Comparative Analysis of Yttria Stabilized Zirconia (YSZ) and Titania Doped YSZ (YZT) Sintered by two Different Routes: Conventional and Microwave Processing
Sonia Mago1, Chetan Sharma1, Payal Sharma1, Kanchan Lata Singh2 and Anirudh Pratap Singh3
and Anirudh Pratap Singh3
1Punjab Technical University, Jalandhar, India.
2DAV Institute of Engineering and Technology, Jalandhar, India.
3Dean, RIC, Punjab Technical University, Jalandhar, India.
Corresponding Author E-mail: kanchan_69@rediffmail.com
DOI : http://dx.doi.org/10.13005/ojc/340541
Article Received on : 12-06-2018
Article Accepted on : 15-09-2018
Article Published : 15 Oct 2018
Besides YSZ (yttria stabilized zirconia) as an appropriate anode material, YZT (titania doped YSZ) is emerging as a material of interest. The undoped YSZ and the doped YSZ i.e. 5 mol % YZT, are synthesized by conventional as well as microwave processing techniques, from the precursors prepared by mixed oxide method and subsequently characterized for a comparative analysis by XRD, Raman spectroscopy, FTIR, SEM and Vickers hardness test to investigate the crystal structure, structural changes taking place during the processing, the surface morphology and the micro hardness of sintered products. Whereas earlier, others have processed the same compositions conventionally, in the present investigation, microwave energy has been exploited for sintering YZT composites. Notable density, phase stabilization, grain growth, hardness and density are observed for microwave sintered products.
KEYWORDS:Micro-hardness; SEM; SOFC; YSZ; YZT; XRD
Download this article as:| Copy the following to cite this article: Mago S, Sharma C, Sharma P, Singh K. L, Singh A. P. Comparative Analysis of Yttria Stabilized Zirconia (YSZ) and Titania Doped YSZ (YZT) Sintered by two Different Routes: Conventional and Microwave Processing. Orient J Chem 2018;34(5). |
| Copy the following to cite this URL: Mago S, Sharma C, Sharma P, Singh K. L, Singh A. P. Comparative Analysis of Yttria Stabilized Zirconia (YSZ) and Titania Doped YSZ (YZT) Sintered by two Different Routes: Conventional and Microwave Processing. Orient J Chem 2018;34(5). Available from: http://www.orientjchem.org/?p=50800 |
Introduction
YZT ceramics, mixed conducting oxides, find multiple applications in photo catalysis, opto-electronic devices, environmental purification, photo eletrochemical solar energy conversion, optical coatings, thermal barrier coatings, dental bio ceramics, Supercritical Water Cooled Reactor Insulator, load bearing implants, oxygen gas sensors, fuel cells etc.1-6
Solid oxide fuel cells (SOFCs) are efficient and environmental friendly energy conversion systems having an electrolyte, an anode, a cathode and the interconnect components.
Introducing yttria dopant to stabilize zirconia provides structural cell support to the electrode. To operate solid oxide fuel cells at high temperatures, single component electrodes have been used so far but mixed conducting ceramics have added advantages.1 Titania doped YSZ i.e. YZT, a mixed conducting oxide, is preferred for SOFC anodes because of stability at high temperatures, matching compatibility with the YSZ, mixed conductivity and improved interfacial bonding with Ni.7,8 Besides, these materials assist in electro catalysis and may affect the phase transformation.4,9 Moreover, for an ideal anode, the property of mixed conduction under fuel conditions is required because this makes the electrochemical reactions possible over the entire TPB in anode cermets.10 The n-type electronic conductivity exhibited by YZT compositions is due to a small polaron-hopping mechanism as a result of electrons hopping between Ti 3+ and Ti4+ ions.7
Here, an economical, faster and energy saving technique11-19 i.e. microwave processing has been explored for preparing YZT composite. Microwave technology has been extensively exploited for sintering ceramic composites. During the microwave sintering, heat is uniformly distributed through dielectric heating. It is noted that microwave sintering involves a smaller firing time and temperature as compared to conventional sintering.4 By the application of microwaves, chemical reactions or thermal processes like sintering can be activated and supported.20,21 Various studies reveal that during the synthesis of BaTiO3 by microwave energy, the non stoichiometry of TiO2 plays an impeccable role in improving coupling of microwaves. Also, while synthesizing PZT, the use of microwave energy enabled PZT to possess a higher dielectric constant relative to that obtained by the conventional treatment. Faster diffusion caused by the microwave field eventually leads to faster processing at comparable temperature according to ‘microwave effect.22
In the current work, undoped YSZ and doped YSZ (5 mol% Titania) are prepared, sintered in an electric furnace at 1400°C for 10 h and in a microwave furnace for 40 min. at the same sintering temperature, and are subsequently characterized by XRD, SEM with Vickers hardness indenter FTIR and Raman Spectroscopy.
Experimental
Materials
The chemical reagents used in the current investigation were obtained from commercial sources with analytical grade purity. We have taken Y2O3 (99.99 % purity, alpha aesar), ZrO2 (99.5 % purity, CDH) and TiO2 (98% purity, CDH) powders as precursor materials. In this work, zirconia balls in the acetone medium were taken for ball milling. An electric furnace of IR radiations was employed for conventional sintering at 1400°C. The microwave furnace used in the study was a modified domestic multimode oven (LG MS-285SD 1200W) of frequency 2.45 GHz and maximum 1.2 KW power output along with inner cavity of dimensions 34.4cm x 34.4cm x 22.5cm. 8% PVA solution was prepared in water which acts as a microwave susceptor.
Synthesis
YZT precursors of two compositions [(ZrO2)0.92(Y2O3)0.08]1-x(TiO2)x where the titania dopant levels (x) used were 0.00 and 0.05, were prepared by mixed oxide method. Y2O3, ZrO2 and TiO2 starting powders were taken in the stoichiometric ratio. The as prepared powders were ball milled for intimate grinding for 6 hours using zirconia balls in the acetone medium put in a polythene bottle. 2% PVA binder was subsequently added to the precursor powders that were consolidated into cylindrical pellets in a cylindrical die of 12.80 mm diameter, under a pressure of about 310 MPa. The conventional sintering of green pellets, five in number, was performed at 1400°C in the electric furnace of for 10 hours. The another set of five green pellets was taken in an alumina crucible, covered with 8% PVA solution and microwave sintered at 1400°C for 40 minutes in order to compare the results to those of conventionally processed ones. Thus, we have four types of samples, two types of samples prepared by the conventional processing and two types of samples prepared by the microwave processing designated as shown in Table 1:
Table 1: Various compositions and abbreviations.
| Composition | Convnetionally Processed | Microwave Processed |
| [(ZrO2)0.92(Y2O3)0.08]1-x(TiO2)x | ||
| X = 0.00 | YSZ_CON | YSZ_MW |
| X= 0.05 | 5 YZT_CON | 5 YZT_MW |
Characterization
The prepared pellets were characterized by different techniques i.e. XRD for the phase identification, SEM for the micro structural analysis with Vickers hardness test for investigating micro hardness, Raman Spectrometer to confirm the crystal alignment and FTIR to study the structural and chemical composition. The equipment used in this study was an X-ray diffractometer (PAN alytical DY 1769, Netherlands) with the CuKα radiation (λ= 1.54Å) to examine the crystal structure of the ceramic composites. The XRD patterns were collected at room temperature over a range of 20°<2θ<80°. Raman spectra were obtained with a Renishaw multichannel spectrometer with a resolution of 1 cm-1. The excitation line 514.53 nm of an Ar-ion laser of power 35 mW was used. The power finally reaching the sample was10%. The spectra were taken at the room temperature. To investigate structural and chemical changes taking place during the processing techniques, a FTIR spectrometer (Agilent Technologies 630), operated in the transmittance mode (%T) was used. Spectra were obtained over 400-4000 cm-1 wave number range. A scanning electron microscope (JSM 6510 LV Japan) was used to characterize the powder microstructure after washing the pellets with water in an ultrasonicator. After polishing and cleaning, the Vickers hardness was measured by a micro hardness tester (OMNITECH, Pune) at a load of about 1 N.
Result and Discussion
Density Measurement
The sintering temperature, the heating rate as well as the sintering time can influence the grain size, density, and mechanical properties of the compacted ZrO2 ceramics.4 Sintering kinetics was studied by heating the samples in a muffle furnace at the chosen temperature of 1400°C. Different pellets of compositions YSZ and 5YZT i.e. 0Ti and 5Ti were treated conventionally and using microwave energy at 1400°C for 10 h as shown in the Table 2.
Table 2: Variation in the density of conventionally and microwave processed YZT with the composition.
| Composition | Conventionally Processed (g/cm3) | Microwave Processed (g/cm3) |
| YSZ | 4.13 | 4.15 |
| 5 YZT | 4.64 | 4.66 |
From these observations in Table 2, it was clear that as sintering time was increased, first the pellets become dense, then with further increase, the density is found to decrease. So a sintering time of about 10 hrs was preferred for further work. It was also observed that the density of microwave processed products is higher than that of the conventionally processed products. The TiO2 and Y2O3 are microwave susceptors therefore ZrO2-Y2O3-TiO2 ternary system which in turn absorbs microwave energy results in better diffusion and higher density of YSZ_MW and 5 YZT_MW compared to those of YSZ_CON and 5 YZT_CON. The reason being an additional driving force in microwave processing, apart from the thermal energy.22 Also the density is increasing with titania content. The increase in density in YZT also suggests that TiO2 is a microwave susceptor and hence a leading factor in enhancement of diffusion causing greater density with the addition of Ti4+ions.23
X-ray Diffraction Analysis
The sintered products were characterized by X Ray Diffractometer (XRD) to explore the phase composition. Fig.1 and Fig. 2 show the XRD patterns of the conventionally treated samples and the microwave treated samples.
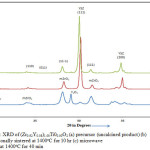 |
Figure 1: XRD of (Zr0.92Y0.08)1.00Ti00.00O2 (a) precursor (uncalcined product) (b) conventionally sintered at 1400oC for 10 hr (c) microwave sintered at 1400oC for 40 min |
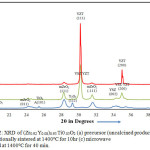 |
Figure 2: XRD of (Zr0.92 Y0.08)0.95 Ti0.05O2 (a) precursor (uncalcined product) (b) conventionally sintered at 1400oC for 10hr (c) microwave sintered at 1400oC for 40 min. Click here to View figure |
Zirconia polymorph is found to exist in the monoclinic, tetragonal and cubic phases. The tetragonal or cubic phase is stabilized by the doping of aliovalent cations. The phase stability of zirconia further depends upon the nature of dopant ions, their concentration and the method of processing. Since ZrO2, TiO2 and Y2O3 are heated together; a number of combinations are expected such as tetragonal YSZ, cubic YSZ, tetragonal YZT, cubic YZT, pyrochlore, zirconium titanate etc. As indicated by XRD analysis, TiO2 insertion has markedly influenced the phase composition and distribution, stimulating the formation of mixed phases in binary and ternary ceramic systems.24 The fluorite structure of cubic zirconia is identified by XRD.8 The cubic phase is identified by (111) peak at an angle of 2θ = 30.1194°C. According to researchers, the microwaves facilitate the formation of a solid solution of yttria and zirconia to obtain pure cubic phase of YSZ.25 The pyrochlore phase is not spotted since peaks of this phase are not detected by XRD. The zirconium titanate phase is also missing. Also, the monoclinic phase is found to decrease significantly for microwave processed products in comparison to the conventionally treated ones. From Fig.2, it is observed that neither anatase nor rutile phase of TiO2 is present in XRD of 5 YZT.26 It suggests that the titania has been able to sustain thermo-chemical reaction during the conventional and the microwave processing, leaving no unreacted TiO2. The outer peaks at around 2θ=34° correspond to tetragonal YZT whereas the middle peak was marked as YSZ/YZT.27 Ti ions replace Zr ions with the octagonal coordination of oxygen. As titanium needs six-coordination, it traps some oxygen vacancies to move away from the 8 coordinated Zr site. This leads to tetragonal phase on insertion of titania.28,29
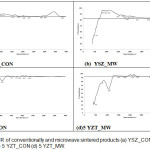 |
Figure 3: FTIR of conventionally and microwave sintered products (a) YSZ_CON (b) YSZ_MW (c) 5 YZT_CON (d) 5 YZT_MW. |
Fig. 3(a), Fig. 3(b), Fig. 3(c) and Fig. 3(d) show FTIR of YSZ_CON, YSZ_MW, YZT_CON, and YZT_MW respectively. Comparing the FTIR of Fig. 3(a), Fig. 3(b), Fig. 3(c) and Fig. 3(d), we find that the number of peaks in FTIR of Fig.3(a) and Fig.3(b) are higher than the peaks in FTIR of Fig.3(c) and Fig.3(d). The above results suggest that the number of phases present in either YSZ_CON or YSZ_MW is more than those of YZT_CON and YZT_MW. This finding is substantiated by the results of the XRD of YSZ_CON and YSZ_MW where it was found that the number of the XRD peaks were more than that of the peaks present in the XRD of YZT_CON and YZT_MW. It has been reported that the peaks due to internal vibrations of ZrO2 molecule in the unit cell come between the range of 514-748 cm-1 including band due to monoclinic zirconia at 740 cm-1.22 Though the peaks of monoclinic phase appear in XRDs of YSZ_CON and YSZ_MW, the peak for monoclinic phase of zirconia could not be deciphered in the FTIR in Fig. 3(a) and Fig.3(b). In Fig.3(c) and Fig.3(d), the symmetric band at around 1100 cm-1 indicated the presence of cubic phase while the presence of the band at around 2100 cm-1 was due to the tetragonal phase. In order to have exact analysis, the Raman Spectra of the above four sample were taken and interpreted.
Raman Spectroscopy
Fig.4 shows the Raman spectra of conventionally and microwave processed, YSZ and 5 YZT samples.
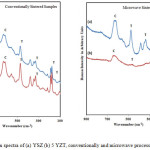 |
Figure 4: Raman spectra of (a) YSZ (b) 5 YZT, conventionally and microwave processed respectively. |
Raman spectra provide more insight into the structural characterization of oxide materials. Different sample preparation condition, fabrication temperature and time may attribute to the difference in phase composition. It is obvious from the fig.4 that the number of peaks in the Raman Spectra of YSZ_CON, YSZ_MW, and 5YZT_CON is large and the spectra are almost similar and their peaks coincide. The Raman Spectra of 5YZT_MW has less number of peaks. The peaks of the spectra of all the compositions have been marked. The peaks in the spectra of YSZ_CON, YSZ_MW, and 5YZT_CON have been marked as m-ZrO2 at ῡ =614, 554, 533, 498,474,379,343, 331, 179 cm-1; t-ZrO2 were marked for the peaks at 474, 263 and 148 cm-1, the peaks due to cubic/tetragonal phase were marked at 630 cm-1. In the Raman spectra of 5YZT_MW, the peaks due to mono clinic phase disappeared and prominent peak of cubic phase remained at 649 cm-1. Weak peaks of t-ZrO2 were marked for the peaks at 474, 263 and 148 cm-1 also remained there.
SEM Characterization
The study of morphologies of the compacted products of YSZ and YZT nano composite was observed with SEM as shown in Fig.5.
 |
Figure 5: SEM micrographs of conventionally and microwave sintered products. |
It was observed by examining the SEM images in Fig.5 that 5 YZT sample is less porous as compared to 0 YSZ sample as this is in accordance with the density results. YZT sample has shown a dense and packed structure at the same sintering temperature. From the average grain size calculated by intercept method given in Table 3, it is clear that the grain size decreases on introduction of titania. Almost no porosity is observed in microwave sintered products while the conventional treatment has been done for 10 hours but the uniformity of the grain is not as good as that of the microwave processed product. The microwave energy enhances the diffusion rate and mobility of oxygen through the ceramics which promotes higher densification.18,30
Vickers Hardness Testing
Hardness is a measure of resistance to penetration by a specified indenter under specified loading conditions. Vickers hardness (H) was measured on a micro hardness tester (OMNITECH, Pune) using the following equation for a load of 1N:
H = 1.854P/d2, where P is the applied load (kg) and d the average diagonal length of the indentation (mm).
The Fig. 6 shows the variation of hardness for microwave processed and conventionally processed samples. The hardness of the 5 YZT_MW is more than that of 5 YZT_CON. Also, the micro hardness of YZT samples is more than that of YSZ samples due to addition of titania. Titania alone has a smaller hardness than YSZ, so the addition of titania should lower the hardness, when it is not diffused into the YSZ structure. But, after sintering, YZT showed a hike in hardness in the samples because of an increased tetragonal phase.3,31
 |
Figure 6: Vickers hardness images for conventionally and microwave sintered products. |
It has already been discussed in the SEM section that the microwave heated samples have less porosity so it can be predicted that the hardness of 0 YSZ_MW and 5YZT_MW samples is greater than 0 YSZ_CON and 5 YZT_CON samples as hardness always increases with decreasing porosity.32 Wang et al investigated that the mechanical strength of a polycrystalline material can be greatly increased by refining its grain size.33 Table 3 shows the decrease in grain size and corresponding increase in micro hardness (in GPa) which is in agreement with the Hall Petch equation according to which hardness varies inversely with the grain size.34-36
Table 3: Variation of grain growth and micro hardness of conventionally and microwave sintered products.
| Composition | Grain Growth (µm) | Vickers Hardness (GPa) |
| 0 YSZ_CON | 3.7 | 1.275 |
| 0 YZT_MW | 3.1 | 1.295 |
| 5 YZT_CON | 0.77 | 5.011 |
| 5 YZT_MW | 0.72 | 5.845 |
It can be inferred from these results that the samples having higher density have higher micro hardness too. In fact, many factors influence the Vickers hardness of ceramics – the deformability within the ceramic and the extrinsic micro structural features like existing phases, porosity, grain size, crystal orientation and boundary formation.37,38
Conclusion
We have successfully synthesized Ti-doped YSZ samples using the mixed oxide method and studied the density, phase, microstructure and micro hardness to evaluate the relative potential of both the conventional as well as the microwave sintering techniques. During the conventional processing, only the thermal energy is the cause of diffusion, while microwave energy is transferred to materials involving molecular interaction with the electromagnetic field thereby modifying the sintering mechanism. This, results in better densification, better micro structure and better hardness of microwave processed products compared to that of the conventionally sintered products. Therefore MW processing proves to be a potentially promising technique to process YZT thereby saving time and energy.
Acknowledgement
We owe gratitude to PITK lab, Kapurthla, NIIT, Jalandhar and Thapar Institute, Patiala for providing characterization facilities.
References
- Nandanwar, R.; Singh, P.; Syed, F.F.; Haque, F.Z. Orient J Chem 2014, 30(4), 1577-1584.
- Romualdez, J.; Kibsey, M.; Huang, X.; Kearsey, R. Turbo Expo 2011, GT2011-45054, Vancouver.
- Kibsey, M.; Romualdez, L.; Huang, X.; Kearsey, R.; Yang, Q. Transactions of the ASME 2011, 133, 122101-1-122101-9.
- Kuo, H.N.; Chou, J. H.; Liu, T.K. Applied Bionics and Biomechanics, 2016, 1-7.
CrossRef - Barrett, F.; Huang, X.; Guzonas, D. J Therm Spray Tech 2013, 22, 734-743.
CrossRef - Rout, P. R. Thesis 2012, National Institute of Technology, Rourkela.
- Colomer, M. T.; Jurado, J.R. Journal of Solid State Chemistry 2002, 165, 79–88.
CrossRef - Skarmoutsos, D.; Tsoga, A.; Naoumidis. A.; Nikolopoulos, P. Solid State Ionics 2000, 135, 439–444.
CrossRef - Haile, S.M. Acta Materialia 2003, 51, 5981–6000.
CrossRef - Tao, S.; Irvine, J.T.S. Journal of Solid State Chemistry 2002, 165, 12–18.
CrossRef - Kochhar, S.P.; Singh, A.P. Asian Journal of Chemistry 2011, 23(8), 3307-3312.
- Sutton, W.H. J. Am. Ceram. Soc. Bull 1989, 68, 376-386.
- Clark, D.E.; Sutton, W.H.; Lewis, D.A. The American Ceramic Society, Ohio, 1997, 80, 61–96.
- Agrawal, D.; Cheng, J.; Fang, Y.; Roy, R. J. Am. Cer. Soc. 2005, 205-228.
- Singh, K.L.; Singh, A.P.; Kumar, A.; Sekhon, S. Advanced Processing and Manufacturing Technologies for Nanostructured and Multifunctional Materials 2014, 1-12.
- Ohji, T.; Singh M.; Mathur, S. Advanced Processing and Manufacturing Technologies for Nanostructured and Multifunctional Materials 2015, 35(6).
- Bodhak, S.; Bose, S.; Bandyopadhyay, A. J. Am. Ceram. Soc. 2011, 94(4), 1281-88.
CrossRef - Furukawa, M.; Horita, Z.; Nemoto,M.; Valiev, R.Z.; Langdon, T.G. Acta Mater. 1996, 44 (11), 4619-4629.
CrossRef - Mago, S.; Singh, K.L.; Singh, A.P. Journal of Nanoscience, Nanoengineering & Applications 2013, 3, 7-15.
- Reisel, A.D.; Schops, S.; Lenk, A.; Schmutzler, G. Advanced Engineering Materials 2007, 9(5).
- Sharma, C; Singh, K; Singh, A; Naithani, V; Sharma, P; Mago, S; Chadha, R. Microwave and conventional processing of niobium and manganese doped lanthanum germanate based apatites, 2018.
- Singh, A.; Kaur, N.; Kumar, A.; Singh, K. J. Am. Ceram. Soc 2007, 90(3), 789-796.
CrossRef - Mago, S.; Sharma, C.; Mehra, R.; Pandey, O.P.; Singh, K.L.; Singh, A.P. Materials Chemistry and Physics 2018 (Accepted DOI: 10.1016/j.matchemphys.2018.06.026).
CrossRef - Khaskhoussi, A.; Calabrese, L.; Bouaziz, J.; Proverbio, E. Ceramics International 2017, 43, 10392–10402.
CrossRef - Colomer, M. T.; Moreno, S. D.; Boada, R.; Maczka, M.; Chaboy, J. Physical Review B 89:094–101 2014, 1-11.
- Traqueia, L.S.M.; Pagnier, T.; Marques, F.M.B, J. Eur. Ceram. Society 1997, 17 (8)1019-1026.
CrossRef - Colomer, M.T.; Guglieri, C.; Moreno, S.D.; Maczka, M.; Boada, R.; Chaboy, J. Journal of Alloys and Compounds 2016, 689, 512-524.
CrossRef
- Colomer M.T.; Maczka, M. J. Solid State Chem. 2011, 184, 365-372.
CrossRef - Feighery, A.; Irvine, A.; Fagg, D.; Kaiser, A., J. Solid State Chem 1999, 143, 273-276.
CrossRef - Singh,,K. L.; Kumar, A.; Singh, A.P.; Sekhon, S.S. Indian Academy of Sciences, Bull. Mater. Sci. 2008, 31(4), 655–664.
CrossRef - Schaedler, T.A.; Leckie, R.M.; Kramer, S.; Evans, A.G.; Levi, C.G. Journal of American Ceramic Society 2007, 90, 3896-3901.
- Miao, X.; Sun, D.; Hoo, P.; Liu, J.; Hu, Y.; Chen, Y. Ceramics International 2004, 30, 1041-1047.
CrossRef - Wang, N.; Wang, Z.; Aust, K.T.; Erb,U. Acta matall. Matter 1995, 43(2), 519-528.
- Pozar, D.M. Microwave Engineering. 2nd ed. (Toronto: John Wiley and Sons, 2001), 1–49.
- Moulson, A.J.; Herbert, J.M. Electroceramics: materials, properties, applications, 2nd ed. (Toronto: John Wiley and Sons, 2003).
CrossRef - Sparks, M. Ferromagnetic-relaxation theory, (New York, McGraw-Hill, 1964).
- Abden, M. J.; Islam, M. K.; Afroze, J.D. International Journal of Materials Engineering 2014, 4(4), 2166–5389.
- Chevalier, J.; Gremillard, L.; Virkar, A. V.; Clarke, D. R. Journal of the American Ceramic Society 2009, 92(9),1901–1920.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









