Synthesis, Spectroscopic Investigation of Co(II),. Ni(II). and Cu(II) Complexes with 2-meracapto-5-(2,4-dinitrophenyl)-1,3,4-oxadiazole or 2-meracapto-5-((4-(dimethylamino)benzylidene)amino)-1,3,4-thiadiazole Ligands
Saleh A. Ahmed1 and Ahmed S. M. Al-Janabi2
1Department of Chemistry, College of Pharmacy, Tikrit University, Tikrit, Iraq.
2Department of Biochemistry, College of Veterinary Medicine, Tikrit University, Tikrit, Iraq.
Corresponding Author E-mail: dr.ahmed.chem@tu.edu.iq
DOI : http://dx.doi.org/10.13005/ojc/3404011
Article Received on : 17-04-2018
Article Accepted on : 03-06-2018
Article Published : 20 Jul 2018
New Co(II), Ni(II) and Cu(II) complexes with 2-meracapto-5-(2,4-dinitrophenyl)-1,3,4-oxadiazole (IpotH) or 2-meracapto-5-((4-(dimethylamino)benzylidene)amino)-1,3,4-thiadiazole (daptH) ligands, were prepared by treatment two moles of thione ligands with one mole of metal salts in EtOH/Acetone and H2O as a solvents, to afforded octahedral complexes of the types [MX2(k2-IpotH)2] (Where M = Co, Cu, X= Cl and M= Ni , X= NO3 ) or [MX2(k2-daptH)2] (Where M = Co, Cu, X= Cl and M= Ni , X= NO3 ). The thione ligands bonded through the nitrogen atom of heterocylic and sulfur atom of thiol group. The prepared ligands and its complexes were characterization by elemental analysis (CHNM), IR spectroscopy , molar conductivity, magnetic susceptibility, UV-Visible spectroscopy and 1H NMR data.
KEYWORDS:Complex; Oxadiazole; Thione; Thiol; Triazole
Download this article as:| Copy the following to cite this article: Saleh A. AhmedSA Al-Janabi A. S. M. Synthesis, Spectroscopic Investigation of Co(II),. Ni(II). and Cu(II) Complexes with 2-meracapto-5-(2,4-dinitrophenyl)-1,3,4-oxadiazole or 2-meracapto-5-((4-(dimethylamino)benzylidene)amino)-1,3,4-thiadiazole Ligands. Orient J Chem 2018;34(4). |
| Copy the following to cite this URL: Saleh A. AhmedSA Al-Janabi A. S. M. Synthesis, Spectroscopic Investigation of Co(II),. Ni(II). and Cu(II) Complexes with 2-meracapto-5-(2,4-dinitrophenyl)-1,3,4-oxadiazole or 2-meracapto-5-((4-(dimethylamino)benzylidene)amino)-1,3,4-thiadiazole Ligands. Orient J Chem 2018;34(4). Available from: http://www.orientjchem.org/?p=47464 |
Introduction
The reaction of ions with thiones compounds have been the subject of various characterization as these chemical compounds contain active groups [NH-CS ↔ -N=C(SH)], and are helpful parent compounds for S including peer of heterocyclic bases.1-5 The ( thiadiazole or oxadiazole )-thione ligands have many coordinate model with metal ions, such as S, N as monodentate, or Sand N as chelate bidentate, N, S-bridging.3-6 The heavy metals such as Pt(II), Pd(II) and Ag(I) were interacted with nitrogen and sulfur donor atoms have been known for its anticancer against with possibility to improve metal-based drugs.1-4 The coordination properties of N and S including heterocyclic ligands, such as triazoles, oxadiazoles, is an emerging and rapidly developing area of research.5-14
Recently, many research works involving M(II) ions with thiones have been published.5-20 In the present work, I report the preparation of Co(II), Ni(II) and Cu(II) complexes with new thione ligands.
Experimental
Methods and Materials
The MX2 (Where M = Co, Cu, X= Cl and M= Ni , X= NO3), and organic compounds were s provided from Sigma-Aldrich, Fluka, or BDH companies, and used without further purification, Melting points were measured on an on SMP40 / Stuart Company, The conductivity of 10-3 M of DMSO or DMF solutions of prepared complexes were measured at 25oC using Digital conductivity meter. The infrared spectra of prepared ligands and its complexes were recorded with KBr disc in the 4000 – 400 cm-1 range on Shimadzu 8400S FTIR Spectrophotometer. The Ultra violet visible data of prepared compounds were measured on Shimadzu UV-1800 Spectrophotometer in 200-1100nm using DMSO as a solvent. Magnetic properties were carried out at 25oC by Bucker BM6 instrument applying Faraday method. CHN element contents were determined by Eurovectro EA 3000/ Italy. The 1HNMR spectra were recorded on Bucker / 300MHz spectrometer with DMSO-d6 as solvent and Me4Sias internal reference. Metal content was determined on Shimadzu SMP30.
Preparation of ligands
Preparation of 2-meracapto-5-(2,4-dinitrophenyl)-1,3,4-oxadiazole (IpotH)
A gradual addition of CS2 (0.076g; 0.001mol) to a mixture of 2,4-dinitrobenzohyrazide (2.200g; 0.001mmol) and KOH (0.560g; 0.001mol) in EtOH (10ml) with stirring. The mixture was refluxed for 20h. The solvent was evaporated to half under vacuum , and ice to the reduced mixture was added, and followed by add of conc. HCl with cooling, and left at room temperature to complete the precipitate process. The yellow ppt. was filtered off, rinsed with H2O and dried in oven under vacuum (Yield: 74%, m.p. 170-173oC) (Scheme 1).
 |
Scheme 1: Preparation of IpotH ligand
|
Preparation of 2-meracapto-5-((4-(dimethylamino)benzylidene)amino)-1,3,4-thiadiazole (daptH)
To an ethanoic solution of 2-mercapto-5-amino-1,3,4-thiadiazol (1.500g; 0.011mol) in EtOH (10ml), a solution of 4-(dimethylamino)benzaldehyde (1.640g; 0.011 mol) in EtOH(10ml) and some drops of CH3COOH (glacial) was added with stirring. The orange solution was refluxed for 4h and cooled on ice bath, the orange ppt. was formed. The product was filtered and rinsed with EtOH (5ml) and Et2O (5ml), and dried in oven under vacuum (Yield: 75%, m.p. 225-226oC) (Scheme 2).
 |
Scheme 2: Preparation of daptH ligand |
General preparation of complexes (1-3)
A solution of IpotH ligand (0.29 g, 1.10mmol) in EtOH/Acetone (1:1) (15 ml) was added to (0.505mmol) of the MX2 (Where M = Co, Cu, X= Cl and M= Ni , X= NO3 ) dissolved in distal water (10 ml). The mixture was refluxed for 2 h. The ppt. afforded was filtered off, rinsed with distal water, and dried in oven under vacuum.
General preparation of complexes (4-6)
A solution of daptH ligand (0.20 g, 0.074mmol) in EtOH/Acetone (1:1) (15 ml) was added to (0.037mmol) of the MX2 (Where M = Co, Cu, X= Cl and M= Ni , X= NO3 ) dissolved in distal water (10 ml). The mixture was refluxed for 3 h. The ppt. produced was filtered off, rinsed with distal water, and dried in oven under vacuum.
Results and Discussion
Synthesis and Characterization of IpotH and its Complexes
Reaction of two moles of IpotH ligand with one mole transition metal MX2 (Where M = Co, Cu, X= Cl and M= Ni , X= NO3) in distal water gave the complexes of the type [MX2(k2-IpotH)2], where M= Co (1), X = Cl; Ni (2), X= NO3; Cu (3), X = Cl, (See. scheme 3). The result complexes are stable in air at room temperature, and are soluble in DMSO and DMF. but insoluble in MeOH, EtOH, acetone, CHCl3 …. etc. The IpotH ligand has been investigated by CHN., 1H NMR, .UV and IR techniques, Where as its complexes have been investigation by molar conductivity, CHNM, UV-visible, and IR techniques.
 |
Scheme 3: Preparation of IpotH complexes (1-3) |
A 10-3M of DMSO and DMF of complexes solutions was prepared and the conductivity of at 25°C were recorded and listed in Table 1.The values of conductivity of the prepared complexes refer that these complexes are non-electrolytes.21
The IpotH ligand is bonded towards the metal ions as bidentate through the N and S atoms to give octahedral arrangement around metal ions.
The 1H nmr spectrum of IpotH in DMSO-d6 (Fig. 1) shows a singlet peak at dH= 4.401ppm due to the proton of thiol group, whereas the protons of phenyl ring appeared as unsolved multiplets within d (6.81-7.87) ppm range. The peak of H-phenyl ring represents three protons, while the SH peak represents one proton, as indicated from the integration values under each signal.
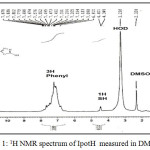 |
Figure 1: 1H NMR spectrum of IpotH measured in DMSO-d6
|
IR spectrum of 2-meracapto-5-(2,4-dinitrophenyl)-1,3,4-oxadiazole (IpotH) (Fig 2) shown a bands at ( 3093, 2538, 1627, 1552, 1380)cm-1 assigned to n(=C-H), n(S-H) , n(C=N), n(C=C), and n(NO2).
Infrared spectra of the of complexes [MX2(k2– IpotH)2] display a strong band at (1568-1594)cm-1 due to the n(C=N) this bond are shifted to low frequency from that of the free ligand which appeared at (1627) cm-1 This indicating that IpotH ligand bounded through the nitrogen atom with metal ions.8,17,22 And the n(S-H) was shifted to lower frequency compared with the free ligand.
The spectra also showed new bands at (483-508) cm-1 and (435-568) which due to n(M-S) and n(M-N) cm-1 respectively,8,17,19,23,24 other IR bands are listed in Table 2.
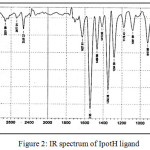 |
Figure 2: IR spectrum of IpotH ligand |
Synthesis and Characterization of [MX2(k2– daptH)2] Complexes
The refluxed two moles of daptH ligands in mixture of EtOH and acetone with one mole of transition metal MX2 (Where M = Co, Cu, X= Cl and M= Ni , X= NO3 ) in distal water gave the complexes of the type [MX2(k2-daptH)2], where M= Co (1), X = Cl; Ni (2), X= NO3; Cu (3), X = Cl, (See. scheme 4). All complexes are stable toward air and wetness, and obtained in high yields (over 70%), and dissolved in DMSO and DMF while partial solubility in warm CHCl3 and warm CH2Cl2. The prepared complexes have been investigated by CHNM analysis, IR spectra, conductivities measurements, and 1H NMR for free ligand.
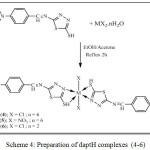 |
Scheme 4: Preparation of daptH complexes (4-6) |
The molar conductance values refer to that these complexes are non-electrolytes. The daptH ligand is coordinated with metal ions as bidentate through the N and S atoms to give octahedral arrangement around metal ions.
The 1H nmr spectrum of daptH in DMSO-d6 (Fig. 3) shows a singlet peak at dH= 3.024ppm due to the proton of methyl group, and this peak represents six protons. and the proton of thiol group appeared as broad singlet at dH= 3.792ppm. The protons of phenyl ring appeared at d6.06ppm as doublet with coupling to the neighboring protons (3JH-H= 8.40Hz) assigned to the H2,2′ proton, and a doublet at d7.59ppm with (3JH-H= 8.40Hz) for the H1,1′ proton. Whereas the protons of HC=N group appeared singlet at 8.212ppm.
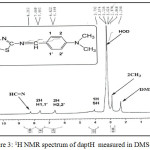 |
Figure 3: 1H NMR spectrum of daptH measured in DMSO-d6 |
IR spectrum of 2-meracapto-5-((4-(dimethylamino.)benzylidene)amino)-1,3,4-thiadiazole (daptH) (Fig. 4)shown a bands at (3091, 2906, 2549, 1691, 1650, 1529, 1460) cm-1 assigned to n(=C-H), n(C-H)aliph., n(S-H)., n(C=N)., n(C=N) ring, n(C=C), and n(C-N).
IR spectra of the of complexes [MX2(k2-daptH)2] showed a strong band at (1587-1614)cm-1 due to the n(C=N) in heterocyclic ring this bond are shifted to low frequency from that of the IpotH ligand which appeared at (1650) cm-1 this refer to that IpotH ligand bounded through the nitrogen atom with metal ions.8,17,22 And the n(S-H) was shifted to lower frequency , this mean the metals ions coordinated through S atom. Other IR bands of the prepared complexes are registered in Table 2.
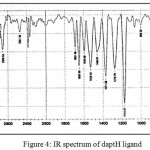 |
Figure 4: IR spectrum of daptH ligand |
Electronic Spectra
The solution of the electronic measurements were prepared in using DMSO as a solvent in 10-3 M are given in Table 3. The UV-Vis. spectra of the prepared ligands IpotH and daptH showed two bands for each ligand, at 28751 and 32258 for the IpotH and at 26385 and 32154 for the daptH which assigned to the n→π* and π→π* respectively. The spectra for all prepared complexes are showed a similar transition as showed in the free ligands it also showed the transitions of the metal d orbitals.21,26
In the [CoCl2(k2-IpotH)2] spectrum, the band appeared at 9814, 10384, and 18832 cm-1 which due to 4A2 → 4T2(F), 4A2 → 4T1(F), 4A2 → 4T1(P), transition respectively. The Co(II) complex showed the μeff value 5.1(B.M), indicating octahedral geometry around the center atom. Whereas in the [Ni(NO3)2(k2– IpotH)2] spectrum, the band appeared at 9425, 11328, and 14728 cm-1 which due to 3A2 → 3T2(F),. 3A2 → 3T1(F),. 3A2 → 3T1(P), transition respectively. Furthermore, octahedral geometry for Ni(II) is also supported by its magnetic moment μeff value 3.1(B.M). The other spectroscopic and physical data are in full agreements with the proposed formulations.25,26
Table 1: Color., Yield, m.p., and CHNM of prepared ligands and its complexes.
| Seq. | Compounds | Color |
Yield (%) |
m.p.(°C) |
Molar conductivity (DMSO/DMF) 10-3M: Ω-1 mol-1 cm-1) |
μeff (B.M) |
Elemental Analysis Found (Calc.)% |
|||
|
C |
H |
N |
M |
|||||||
| 1 | IpotH |
Light yellow |
74 |
170-173 |
– |
– |
35.67 (35.83) |
1.36 (1.50) |
20.69 (20.89) |
|
| 2 | [CoCl2(k2– IpotH)2] |
Light violet |
79 |
181-185 |
10 / 19 |
5.1 |
28.67 (28.84) |
1.34 (1.21) |
16.57 (16.82) |
8.72 (8.85) |
| 3 | [Ni(NO3)2(k2– IpotH)2] |
Green |
86 |
215-218 |
12 / 18 |
3.1 |
26.89 (26.72) |
1.28 (1.12) |
19.65 (19.48) |
8.27 (8.16) |
| 4 | [CuCl2 (k2– IpotH)2] |
Brown |
76 |
161-165 |
14 / 23 |
1.7 |
28.79 (28.65) |
1.43 (1.20) |
16.87 (16.70) |
9.27 (9.47) |
| 5 | daptH |
Orange |
75 |
225-226 |
– |
– |
49.43 (49.98) |
4.31 (4.58) |
20.86 (21.19) |
– |
| 6 | [CoCl2(k2– daptH)2] |
Dark brawn |
81 |
265-267 |
6 / 15 |
4.8 |
39.86 (40.12) |
3.28 (3.67) |
16.71 (17.02) |
8.83 (8.95) |
| 7 | [Ni(NO3)2(k2– daptH)2] |
Light green |
72 |
292-294 |
10 / 17 |
2.8 |
37.23 (37.14) |
3.59 (3.40) |
19.58 (19.69) |
8.11 (8.25) |
| 8 | [CuCl2 (k2– daptH)2] |
Green |
88 |
253-256 |
15 / 22 |
1.5 |
39.52 (39.84) |
3.24 (3.65) |
16.66 (16.90) |
9.45 (9.58) |
Table 2: IR data (cm-1) of the prepared ligands and their complexes.
| Complexes |
v(C-H)ar |
v(C-H)aliph |
v(S-H) |
v(C=N) |
v(NO2) |
v(M-N) |
v(M-S) |
| IpotH |
3093 |
– |
2538 |
1627 |
1350 |
– |
– |
| [CoCl2(k2– IpotH)2] |
3058 |
– |
2521 |
1568 |
1371 |
512 |
443 |
| [Ni(NO3)2(k2– IpotH)2] |
3052 |
– |
2498 |
1591 |
1360 |
483 |
423 |
| [CuCl2 (k2– IpotH)2] |
3089 |
– |
2518 |
1594 |
1360 |
568 |
508 |
| daptH |
3091 |
2906 |
2552 |
1650 |
– |
– |
– |
| [CoCl2(k2– daptH)2] |
3058 |
2889 |
2528 |
1587 |
– |
521 |
438 |
| [Ni(NO3)2(k2– daptH)2] |
3098 |
2923 2858 |
2531 |
1614 |
– |
498 |
421 |
| [CuCl2 (k2– daptH)2] |
3052 |
2968 2887 |
2518 |
1607 |
– |
508 |
427 |
Table 3: Electronic spectral data of prepared ligands and its complexes in DMSO.
| Seq. | Compounds |
Absorption bands cm-1 |
Assigned transition |
| 1 | IpotH | 32258, 28751, | n→π* , π→π* |
| 2 | [CoCl2(k2– IpotH)2] | 30675, 27932,9814, 10384, 18832 | n→π* , π→π*,4A2g → 4T2g(F) ., 4A2g→ 4T1g(F) ., 4A2g→4T1g(P) |
| 3 | [Ni(NO3)2(k2– IpotH)2] | 30796, 24938,9425, 11328, 14728 | n→π* , π→π*,3A2g→ 3T2g(F), .3A2g→ 3T1g(F), .3A2g→ 3T1g(P), |
| 4 | [CuCl2 (k2– IpotH)2] | 31546, 23578,11699 | n→π* , π→π*,2Eg → 3T2g |
| 5 | daptH | 26385, 32154 | n→π* , π→π* |
| 6 | [CoCl2(k2– daptH)2] | 33,180, 25,4528,265, 9,346, 15,455 | n→π* , π→π*,4A2g → 4T2g(F), . 4A2g→ 4T1g(F), .4A2g→ 4T1g(P) |
| 7 | [Ni(NO3)2(k2– daptH)2] | 30581, 24631,9434, 10358, 13661 | n→π* , π→π*,3A2g → 3T2g(F), . 3A2g → 3T1g(F), .3A2g → 3T1g(P), |
| 8 | [CuCl2 (k2– daptH)2] | 29326, 22897,11628 | n→π* , π→π*,2Eg → 3T2g |
Conclusions
In summary, we have synthesized IpotH ligand by refluxing CS2 and 2,4-dinitrobenzohyrazide and KOH or daptH ligand by condensation of 2-mercapto-5-amino-1,3,4-thiadiazol with 4-(dimethylamino)benzaldehyde and their complexes with Co(II), Ni(II) and Cu(II) to afford mononuclear complexes of the type [MX2(k2-thione)2],(M(II) = Co, Ni and Cu). The thione ligand bonded as bidentate chelating through the nitrogen and sulfur atoms to give octahedral geometry around the metal ions with 1:2 (metal: ligand) stoichiometry. The prepared ligands and its complexes were characterization by CHNM analysis, IR spectroscopy , molar conductivity, magnetic susceptibility, UV-Visible and 1H NMR data.
References
- per, E. S. Coord. Chem. Rev., 1994; 129, 91-159.
CrossRef - RaRaper, E. S. Coord. Chem. Rev., 1985; 61, 115-184.
CrossRef - Raper, E. R. Coord. Chem. Rev., 1996; 153, 119-225.
CrossRef - Isab, A. A., Wazeer, M. I., Ashraf, M. W. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2009; 72(1), 218-221.
CrossRef - Bharty M.K., Dani,R.K., Kushawaha,S.K., Singh, N.K., Kharwar, R.N., Butcher R.J., Polyhedron, 2015; 88, 208-211.
CrossRef - Bharati, B. P., Bharty, M.K., Dani, R.K., Singh, S., Singh,N.K., Polyhedron, 2013 ; 54, 131-139.
CrossRef - Al-JanabI A. S. M, Abdullah B. H, Al-Jibori S. A. , Orient J Chem. 2009; 25(2), 277-286.
- Al-Janabi A. S. M, Al-Soumadaiy G. A, Khear-Allah B. A. , Orient J Chem. 2011; 27(4), 1465-1473.
- Al-Janabi A. S. M, Ahmed S. A. O. , Orient J Chem. 2011;27(4), 1563-1571.
- Al-Janabi A. S. M., Tikrit J. of Pure Science, 2015; 20 (4), 67-72.
- Al-Janabi A. S. M., Tikrit J. of Pure Science, 2016; 21 (7), 95-100.
- Al-Janabi A. S. M, Ahmed. S. A, Ahmed S. A. O., Kirkuk University Journal /Scientific Studies, 2017; 12(2), 9-25.
- Amin, O. H., Al-Hayaly, L. J., Al-Jibori, S. A., Al-Allaf, T. A.K., Polyhedron, 2004; 23, 2013-2020.
CrossRef - Al-Jibori, S. A., Khaleel,T. F., Ahmed, S. A.O., Al-Hayaly, L. J., Merzweiler, K., Wagner, C., Hogarth, G., Polyhedron, 2012; 4120-24.
- Bell, N. A., Clegg, W., Coles, S. J., Constable, C. P., Harrington, R. W., Hursthouse, M. B., Light, M. E., Raper, E. S., Sammon, C., Walker,M. R., Inorg. Chim. Acta., 2007; 357(11) 2091-2099.
- Nam, H. J. Lee, H. Noh, D.Y. Polyhedron, 2004; 23(1)115-123m.
CrossRef - Evangelinou, O. Hatzidimitriou, A.G. Velali, E. Pantazaki, A.A. Voulgarakis, N. Aslanidis, P., Polyhedron, 2014; 72, 122-129.
CrossRef - Nawaz, S., Isab,A. A., Merz, K., Vasylyeva, V., Metzler-Nolte, N. Saleem, M., Ahmad, S., Polyhedron, 2011;30,1502-1506.
CrossRef - Kaltzoglou, P., Cox, J., Aslanidis, P., Inorg. Chim. Acta., 2005; 358(11) 3048-3056.
CrossRef - Kubicki, M., Hadjikakou, S.K., Xanthopoulou, M. N., Polyhedron, 2001; 20(17),2179-2185.
CrossRef - Geary, W.J., Coord. Chem. Rev.,1971; 71-122, 1971
- Silverstein, R.M., Webster, F.X. ”Spectrometric Identification of Organic Compounds”, 6th ed., John Wiley & Sons, New York, NY, USA,1997.
- Jensen, K. A. Nrelsen, P. H., Acta. Chem. Scand., 1963; 17, 1875-1885.
CrossRef - Nakamoto, K., ”Infra-Red and Raman Spectra of Inorganic and Coordination Compounds” , 4th ed. John Wiley & Sons, New York, 1986.
- Abuhijleh, A.L., Woods, C., Ahmed, I.Y., Inorg. Chim. Acta, 1991; 190, 1.
CrossRef - Cotton, F.A., Wilkinson, G., ”The elements of first row transition series,. In: Advanced Inorganic Chemistry”, 3rd ed., Wiley, New York, 1992.

This work is licensed under a Creative Commons Attribution 4.0 International License.









