Synthesis of Nanobentonite as Heavy Metal Adsorbent with Various Solvents
Makmur Sirait1 , Saharman Gea2, Nurdin Bukit1, Nurdin Siregar1 and Ceria Sitorus1
, Saharman Gea2, Nurdin Bukit1, Nurdin Siregar1 and Ceria Sitorus1
1Department of Physics, Faculty of Mathematics and Natural Sciences, Universitas Negeri Medan, Jl. Willem Iskandar Pasar V, Medan 20221, Indonesia.
2Department of Chemistry, Faculty of Mathematics and Natural Sciences, University of Sumatera Utara, Jl. Bioteknologi No 1, Medan 20155, Indonesia.
Corresponding Author E-mail: maksir@unimed.ac.id
DOI : http://dx.doi.org/10.13005/ojc/3404020
Article Received on : 27-07-2018
Article Accepted on : 02-08-2018
Article Published : 28 Aug 2018
The nanobentonite has been synthesized from natural bentonite taken from Tapanuli Utara, Indonesia using coprecipitation method with various solvents (HCl, H2SO4, and HNO3). Its properties as a metal adsorbent were investigated by Atomic Adsorption Spectrophotometry. X-Ray Diffraction analysis revealed that the bentonite produced is in nanometer scale. The characterization results obtained from the SiO2single phase with highest dhkl was at millier index (101) with 2 of 21.9o, 22.0o, 22.07o respectively. The results of Microscope-Scanning Electron Energy analysis of nanobentonite dispersion indicated a reduction in agglomeration and finer nanobentonite surface. The Surface Area Analyzer results showed the SBET nanobentonite for solvent variation of HCL, H2SO4, and HNO3 respectively were 731.76 m2/g, 868.11 m2/g, 493.97 m2/ g. Lastly, Atomic Adsorption Spectrophotometric test showed that the optimal absorption of the metal content possessed by variety of HCl and nanobentonites with adsorption power of 91.16% for Pb, 76.39% for Cu, and 82.74% Co.
KEYWORDS:Coprecipitation; Metal Adsorben; Nanobentonite; Various Solvent
Download this article as:| Copy the following to cite this article: Sirait M, Gea S, Bukit N, Siregar N, Sitorus C. Synthesis of Nanobentonite as Heavy Metal Adsorbent with Various Solvents. Orient J Chem 2018;34(4). |
| Copy the following to cite this URL: Sirait M, Gea S, Bukit N, Siregar N, Sitorus C. Synthesis of Nanobentonite as Heavy Metal Adsorbent with Various Solvents. Orient J Chem 2018;34(4). Available from: http://www.orientjchem.org/?p=48894 |
Introduction
Pollution of the aquatic environment by heavy metals becomes a serious problem in public health and a very important environmental issue.1 Lead (Pb) is toxic to public health. Lead can cause brain damage, inhibition of growth of children, increased blood pressure and digestion, kidney damage, nerve damage and joint disorder.2 Similarly to copper metal (Cu), it can accumulate in body tissues that can be toxic to humans. The poison generated can result vomiting, burning sensation in the esopagus and stomach, colic, diarrhea, which is then followed by hypotension, liver necrosis and coma.3 Another heavy metal which widely found in aquatic environment and can cause a harmful effect is cobalt. It is a toxic metal that can cause vomiting and nausea, vision problems, heart problems, thyroid damage.4
Recently, various researches that utilize minerals have been addressed to tackle this problem by optimizing the adsorption properties to bind the heavy metals and other contaminants and prevent them from polluting the environment.5-8 Accordingly, one of the mineral that has been used to be an effective heavy metal adsorbent is bentonite. A study reported that nanobentonite can be used as a heavy metal ion adsorption where 90% of the cobalt is absorbed by the nanobentonites with concentration of 25 mg/L and 0.5g/50 mL.9 Zuzana (2012) made the adsorbent of heavy metal ions Pb with nanobentonite where in the activated bentonite, the adsorption capacity for metal ions Pb2+ and Cu2+ ions are 32.68 mg/g and 11.34 mg/g respectively.10
Converting natural bentonite into a nano-scale bentonite can be conducted by using coprecipitation method.11 This method is proven to be effective to result a bigger area of nanobentonite produced. Pawar (2015) reported, with a surface area method of coprecipitation, produced an area of 294 m2/g. From these study, it was found that coprecipitation method is suitable to produce a large surface area that can improve the adsorption capacity of nanobentonite.12
In this particular study, the use of nanobentonite as a heavy metal adsorbent will be varied with various solvents, namely HCl, H2SO4, and HNO3. This is considered to enhance the optimum adsorption capacity, a large surface area, and the size of the smallest nanobentonite. In terms of the adsorption of heavy metal ions, the metal that will be examined is Pb, Cu and Co. Lastly, preparation of nanobentonite from natural bentonite was conducted to improve the quality of nanobentonite as adsorbent that can trap heavy metal ions.
Materials and Methods
Natural bentonite used in the study derived from Pahae, North Tapanuli, North Sumatra, Indonesia. Nanobentonite from natural bentonite was synthesized with the copresipitation method using 200 mL of 12 M HCl as a solvent, stirred for 120 minutes at 300 rpm and at a temperature of 70oC using the magnetic stirrer. As many as 150 mL NH4OH was stirred with a magnetic stirrer for 120 minutes at 300 rpm, 70oC. Then, HCl solution was slowly poured into a solution of NH4OH and stirred at 350 rpm, 70oC for 120 minutes. Distilled water was added to neutralize the solution pH. The solution was dried in the oven for 5 hours at a temperature of 100°C. The dried precipitate was crushed by using mortar to produce nanobentonite. The similar steps were repeated with the H2SO4 and HNO3. Nanobentonite was characterized by Scanning Electron Energy Microscope-Dispertion, X-Ray Diffraction and Surface Area Analyzer. Analysis of nanobentonite adsorption capacity on heavy metal ions was done by preparing waste water with a metal content of Pb 92.50 ppm. Nanobentonite was poured into the waste water (250 ml), then stirred with a magnetic stirrer for 1 hour. The analyzed sample was a solution on four bottles namely A (indicator), B (Addition of HCL solvent nanobentonite), C (addition of H2SO4nanobentonite solvent), and D (addition of HNO3 solvent nanobentonite).
Results
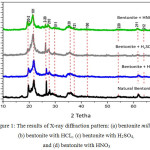 |
Figure 1: The results of X-ray diffraction pattern: (a) bentonite milling, (b) bentonite with HCL, (c) bentonite with H2SO4, and (d) bentonite with HNO3 |
Table 1: Estimated measurements crystalline diameter XRD test.
| Sample | Chrystalline Size (nm) |
| Bentonite powder milling | 80.00 |
| Nanobentonit with HCL | 16.46 |
| Nanobentonit with H2SO4 | 13.60 |
| Nanobentonit with HNO3 | 23.72 |
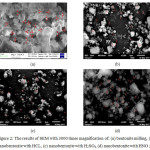 |
Figure 2: The results of SEM with 3000 times magnification of: (a) bentonite milling, (b) nanobentonite with HCL, (c) nanobentonite with H2SO4, (d) nanobentonite with HNO3 |
Table 2: The particle size of bentonite with various solvents.
|
Sample |
Particle Size (nm) |
|
Bentonite powder milling |
121.55 |
|
Nanobentonite with HCL |
42.95 |
|
Nanobentonit with H2SO4 |
33.00 |
|
Nanobentonit with HNO3 |
41.60 |
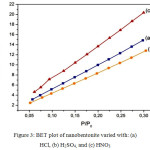 |
Figure 3: BET plot of nanobentonite varied with: (a) HCl, (b) H2SO4, and (c) HNO3 |
Table 3: SBET value for each nanobentonite.
|
Sample |
S BET (m2/g) |
|
Nanobentonite with HCL |
731.76 |
|
Nanobentonit with H2SO4 |
868.11 |
|
Nanobentonit with HNO3 |
493.97 |
Table 4: The metal content of each nanobentonite.
|
Sample |
Pb (ppm) |
Cu (ppm) |
Co (ppm) |
|
Indicator |
92.50 |
54.2 |
64.3 |
|
Nanobentonite with HCL |
8.18 |
12.80 |
11.10 |
|
Nanobentonit with H2SO4 |
60.5 |
16.4 |
18.3 |
|
Nanobentonit with HNO3 |
10.8 |
13.7 |
11.00 |
Discussion
Based on figure 1, the main peak of the index (101) with a second angle 2θ = 22.04o with SiO2 phase. It is also strengthened by the emergence of several specific dhkl peaks with miller indices of (20-2), (2-20), (210), (011) and (120), (100), (200), (212 ). Best spectrum of the XRD analysis results is showed by nanobentonite with H2SO4 solvent which has a higher peak in the phase of SiO2 and trigonal crystalline structure and diameter of 13.60 nm. This result is better than Sirait’s (2017) study performing nanobentonite synthesis having a crystalline size of 35.26 nm.11
Figure 2 shows the morphology of nanobentonite that tends to have a rough spherical shape. The particle size was analyzed by the Scherer equation. From table 2, bentonite particles known to use H2SO4 has the smallest particle size. The size of the smaller particle diameter caused by the reaction of acid (HCl, H2SO4, and HNO3), because in addition to being cleaner for impurities on bentonite particles, it is also destructive to particles so as to make the particle size smaller.
Based on the data obtained by BET analysis, the largest surface area is with H2SO4as solvent at 84.84 m2/g and a surface area of a specific surface area (SBET) is 868.10 m2/g. Pawar (2015) stated that the manufactured of nano bentonite with H2SO4 as solvent obtained the specific surface area of 294 m2/g. It can be concluded that the research results are better than previous Pawar study (2015) because of the larger specific surface area.
The residue of waste water were analyzed by AAS to get the value of adsorption, which is shown in table 4. Moreover, it can be seen in table 3 that the absorption of Pb is more optimal using the HCl and nanobentonite variation (91.16%) compared with the use of nanobentonite obtained using H2SO4(34.60%) and HNO3(88.33%). Cu optimal absorption was obtained using the HCL nanobentonite (76.39%) compared with the use of H2SO4 (69.74%) and HNO3(74.72%) nanobentonite. Cobalt absorption was shown by the HCL nanobentonite (82.74%) compared to metal absorption using H2SO4 (71.54%) but it is comparable with the HNO3 nanobentonite (82.90%).
Conclusions
Nanobentonite particles have been synthesized from natural bentonite from Pahae, South Tapanuli District, North Sumatra, Indonesia, by coprecipitation method. Variations of solvent in coprecipitation method was conducted to obtain three different samples, ie variation of HCL, H2SO4 and HNO3. The XRD results showed that all three samples had a single phase, the SiO2. Nanobentonite with solvent H2SO4 has a smaller particle size with smaller surface area compare to nanobentonite with HCL solvent. This is caused by the silica content which have absorption properties at the nanobentonite solvent H2SO4 smaller but the most optimal absorption occurs in nano bentonite with HCL solvents that absorb Pb 91.16%, which has a surface area of 713.76 m2/g. SEM analysis is suggested for further and more accurate particle size determination. It is also suggested to perform the analysis of adsorbent capacity of this nanobentonite with other heavy metals.
Acknowledgement
Authors would like to thank to Ministry of Research and Higher Education of Indonesia for research funding support via DRPM 2018 funding scheme.
References
- Xue, Y.; Hou, H.; Zhu, S. J. Hazardous Mater. 2009, 162, 391-401
CrossRef - Naseem, R.; Tahir, S. S. Water Res. 2001, 35, 3982-3986
CrossRef - Paulino, A. T.; Minasse, F. A. S.; Guilherme, M. R.; Reis, A. V.; Muniz, E. C.; Nozaki J. J. Colloid Interf. Sci. 2006, 301, 479-487
CrossRef - Rao, G. B.; Prasad, M. K.; Murthy, Ch. V. R. Int. J. Chem. Sci. 2015, 13(4), 1893-1910
- Abas, S. N. A.; Ismail, M. H. S.; Kamal, M. L.; Izhar, S. World Appl. Sci. J. 2013, 28(11), 1518-1530
- Zvinowanda C. M.; Okonkwo, J. O.; Shabalala, P. N.; Agyei, N. M. Int. J. Environ. Sci. Tech. 2009, 6(3), 425-434
CrossRef - Lata, S.; Singh, P. K.; Samadder, S. R. Int. J. Environ. Sci. Technol. 2015, 12, 1461–1478
CrossRef - Ayanda, O. S.; Sodeinde, K. O.; Okolo, P. O.; Ajayi, A. A.; Nelana, S. M.; Naidoo, E. B. Orient. J. Chem. 2018, 34(3), 1233-1239
CrossRef - Shahrani, A. S. S. Alexandria Eng. J. 2014, 53, 205-211
CrossRef - Zuzana, O.; Annamaria, M.; Silvia, D.; Jaroslav, B. Arhiv za technicke nauke. 2012, 7(1), 49-56
- Sirait, M.; Bukit, N.; Siregar, N. AIP Conference Proceedings 1801, 020006 2017, doi: 10.1063/1.4973084
CrossRef - Pawar, R. R.; Lalhmunsiama; Bajaj, H. C.; Lee, S. J. Industrial Eng. Chem. 2016, 34, 213-223
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










