Synthesis and Characterization of K2O/MCM-41 (Mobil Composition of Matter No. 41) from Lapindo Mud by Sonochemical Method for Transesterification Catalyst of used Cooking Oil
Dwi Putra Wijaya1, Wega Trisunaryanti 1 , Triyono1, Kumala Dewi2 and Muhammad Fajar Marsuki3
, Triyono1, Kumala Dewi2 and Muhammad Fajar Marsuki3
1Department of Chemistry, Faculty of Mathematic and Natural Sciences, Universitas Gadjah Mada, Sekip Utara Bulaksumur, Yogyakarta, 55281, Indonesia.
2Faculty of Biology, Universitas Gadjah Mada, Sekip Utara Bulaksumur, Yogyakarta, 55281, Indonesia.
3Faculty of Mathematic and Natural Sciences, State University of Malang, Malang, East Java, 651145, Indonesia.
Corresponding Author E-mail: wegats@ugm.ac.id
DOI : http://dx.doi.org/10.13005/ojc/3404019
Article Received on : 07-05-2018
Article Accepted on : 23-08-2018
Article Published : 26 Aug 2018
The MCM-41 was synthesized using CTAB as a template by sonochemical method and it was charactherized by FTIR, XRD, SAA, and TEM. Potasium was impregnated onto the MCM-41 using potasium acetate salt solution with K+ concentrations of 0.80, 1.35, 1.86, and 2.49 wt.% to produce K2O(1), K2O(2), K2O(3), and K2O(4)/MCM-41 catalysts. The K2O/MCM-41 catalysts were then analyzed by ICP and SAA. Acitivity of catalysts were evaluated in the transesterification reaction of used cooking oil at 50, 60, and 70°C for 120 min. The MCM-41 showed characteristic peaks of 2θ = 2-3°C. The TEM images showed ordered pore distribution with a hexagonal shape. The MCM-41 and K2O(4)/MCM-41 have spesific surface area of 1282.33 and 225.81 m2/g with pore diameter of 30.49 and 30.12 Å, respectively. The highest conversion of methyl ester was obtained from transesterification at 70°C using K2O(4)/MCM-41 catalyst and it was about 79.80 wt.%. Catalyst lifetime of the K2O(4)/MCM-41 for transesterification of used cooking oil was about 15.41 h.
KEYWORDS:Lapindo; Mud; Sonochemical; K2O/MCM-41; Transesterification
Download this article as:| Copy the following to cite this article: Wijaya D. P, Trisunaryanti W, Triyono T, Dewi K. Marsuki M. F. Synthesis and Characterization of K2O/MCM-41 (Mobil Composition of Matter No. 41) from Lapindo Mud by Sonochemical Method for Transesterification Catalyst of used Cooking Oil. Orient J Chem 2018;34(4). |
| Copy the following to cite this URL: Wijaya D. P, Trisunaryanti W, Triyono T, Dewi K. Marsuki M. F. Synthesis and Characterization of K2O/MCM-41 (Mobil Composition of Matter No. 41) from Lapindo Mud by Sonochemical Method for Transesterification Catalyst of used Cooking Oil. Orient J Chem 2018;34(4). Available from: http://www.orientjchem.org/?p=48761 |
Introduction
One of the most potential fuels is biodiesel. Biodiesel research was initiated by Rudolf Diesel, in 1900 invented a gasoline diesel engine based on peanut oil. In its development, it is not only peanut oil that can be converted into biodiesel, but also cooking oil or palm oil can be converted into biodiesel.1 Palm oil has the largest percentage of palmitic acid and oleic acid compared to other oil sources.2 It means that conversion of palm oil will produce more biodiesel.
Palm oil is a daily food ingredient used to cook. During frying process, unwanted complex reactions occur leading to oil damage such as oxidation, thermal polymerization, hydrolysis, and pigment degradation.2 Used cooking oils, which are used repeatedly, may contain peroxide compounds, hydroperoxides, polymers, hydrocarbons, and other carcinogenic compounds.3 The process of converting used cooking oil into biodiesel by alcohol or methanol requires a catalyst.4 This catalysts are usually homogeneous base catalysts such as NaOH and KOH. However, the use of homogeneous base catalysts on conversion of used cooking oil usually produce a corrosive waste and the catalyst is difficult to be separated. So, it needs more effective catalysts which are easily separated and has a great activity for transesterification process such as heterogeneous base catalysts.
Many researchers reported that K2O/MCM-41 is one of the heterogeneous base catalysts that can be used in transesterification process. It can be prepared by modification of MCM-41 with a potassium salt solution and it provides an alkaline characteristic for MCM-41 in transesterification process. MCM-41 is a mesoporous material with a pore diameter of 2-50 nm, hexagonal-shaped pores, and silica as its counterpart, therefore a silica-rich raw material is required in MCM-41 preparation.5,6 Some researcher reported that Lapindo mud has a high silica content of 54.10 wt.% making it to be suitable as a source of silica in the synthesis of MCM-41.7,8 The synthesis of MCM-41 has been done in various ways such as synthesis using hydrothermal and non-hydrothermal method. Hydrothermal method requires a long time heating.9 In other hand, non-hydrothermal methods does not require a long time heating, so the process is more efficient.5 The MCM-41 synthesized using non-hydrothermal method produced MCM-41 with a lower order pores than that of hydrothermal method. One of alternative non-hydrothermal method in synthesis of MCM-41 is sonochemical method. The sonochemical method uses ultrasonic waves over a short period of time and it will affect the crystallization and reaction process.
In this work, synthesis of MCM-41 was carried out by sonochemical method using silica from Lapindo mud. The MCM-41 was then impregnated with potassium under variation of content of 0.80, 1.35, 1.86, and 2.49 wt.% produced K2O(1), K2O(2), K2O(3), and K2O(4)/MCM-41 catalysts. These catalysts were used in transesterification of used cooking oil under the variation of temperature of 50, 60, and 70°C
Experimental
Materials
Hydrochloric acid (HCl), sodium hydroxide (NaOH), sulfuric acid (H2SO4), cetil trimethyl ammonium bromide (CTAB), silver nitrate (AgNO3), potassium acetate (CH3COOK), and methanol (CH3OH) were purchased from Merck. Lapindo mud was collected from Sidoarjo Regency, East Java, Indonesia.
Instrumentation
The equipments used in this research were X-ray Fluorescence (XRF, PAN analytical Minipal 4), Fourier Transform Infrared spectrometer (FTIR, Shimadzu Prestige 21 model), Transmission Electron Microscope (TEM, JEOL JEM-1400), X-ray Diffractometer (XRD, Rigaku Miniflex 600), Surface Area Analyzer (SAA, Quantachrome Nova Win version 11.0), Inductively Coupled Plasma Spectrometry (ICPE-9820), and Gas Chromatography Mass Spectrophotometer (GC-MS, Shimadzu QP2010S).
Extraction of silica from Lapindo mud
Extraction of silica from Lapindo mud was carried out by following Fajar procedure10 with slight modification. The Lapindo mud was sieved to 100 mesh, washed with aquadest, and dried at 100°C for 24 h. The mud was refluxed with HCl 6 M solution at 90°C for 3 h and washed with water up to pH 7. The mud was then refluxed with NaOH 6 M solution at 90°C for 16 h. The filtrate was separated and added with HCl 3 M solution until pH 8. The formed precipitate was filtered, washed with aquabidest, and dried at 80°C for 24 h. The extracted silica was analyzed by XRF.
Synthesis of MCM-41
Non-hydrothermal synthesis of MCM-41 was carried out by following Ifah4 procedure. 1.5 g of silica was dissolved in 50 mL of aquabidest containing 3 g NaOH and aging for 2 h. The CTAB was dissolved in 45 mL aquabidest and stirred for 30 min at 50°C. The Na2SiO3 solution was then dropwise added into the CTAB solution while the CTAB solution was stirred slowly until the solution be homogenous. After the addition of Na2SiO3 solution, H2SO4 1M solution was dropwise added into the mixture to reach pH 11 until white gel formed and the mixture was stirred for 1 h. The white gel solution was sonicated for 150 min. The precipitate of the sonicated gel solution was then filtered and washed with aquabidest. The solid was then dried at 100°C for 5 h. The MCM-41 was calcined at 540°C for 5 h. The MCM-41 before and after calcination were analyzed using XRD, FTIR, TEM, and SAA.
Synthesis of K2O/MCM-41 catalyst
The K2O/MCM41 was prepared by following Mahardika5 procedure. The synthesis was performed by adding 0.80 wt.% of potassium acetate salt to MCM-41 with 100 mL of aquabidest and strirring the mixture at 60°C for 180 min. The solid was separated from the filtrate and then dried at room temperature. The solid was calcined at 550°C for 360 min to produce K2O(1)/MCM-41. Variation of K+ concentration was made in the same way on 1.35, 1.86, 2.49 wt.% of K+ impregnated onto MCM-41 to produce K2O(2), K2O(3), and K2O(4)/MCM-41 catalysts, respectively. The catalysts were analyzed by ICP and SAA.
Transesterification of used cooking oil
The K2O/MCM-41 catalyst activity test was conducted by transesterification of used cooking oil. The transesterification process is carried out by refluxing methanol, used cooking oil and the catalysts. The transesterification was carried out at 50, 60, and 70°C for 2 h with mole ratio of methanol/used cooking oil of 15/1. The amount of catalyst used for each transesterification process was about 10 wt.% towards the used cooking oil. After refluxing process, the mixture was separated using a centrifugator at 2000 rpm for 20 min. The ester product was separated and then washed with warm aquabidest. The ester product was then analyzed using GC-MS. The catalyst life-time determination was performed by reusing the catalyst for three times (2 h for each run). The used catalyst was washed with aquabidest and methanol and it was then dried at 100°C for 4 h before it was reused.
Results and Discussion
Extraction of Silica
The silica extraction from Lapindo mud were carried out using 3 steps by washing with H2O, reflux with HCl 6 M solution and NaOH 6 M solution. The purity of silica extracted from Lapindo mud was about 96.70 wt.%. The chemical composition of silica extracted from Lapindo mud is presented in Table 1. This chemical composition data was obtained using XRF.
Table 1: Chemical composition of silica extracted from Lapindo mud
|
Compounds |
Yield (wt.%) |
|
SiO2 |
96.7 |
|
Al2O3 |
– |
|
CaO |
1.30 |
|
TiO2 |
0.37 |
|
Fe2O3 |
0.83 |
|
Others |
0.80 |
Characterization of MCM-41
Fig. 1 shows the FTIR spectra of MCM-41. An absorption peak at 1095 cm-1 in Fig.1(b) indicates the vibration of Si-O-Si group.10 The absorption peaks at 1481, 2924 and 2854 cm-1 in Fig. 1(a) refers to the vibration of CH2 and CH3-N+ groups in CTAB chain.6 The FTIR spectra of the MCM-41 before and after the calcination treatment are showed in Fig. 1(c) and 1(d), respectively. A sharp absorption peak at 1094 cm-1 corresponds to the symmetry vibration of Si-O-Si.8 The widening absorption peak at 3000-3700 cm-1 marks the vibration of the OH from silanol groups or physically absorbed H2O molecules.11 The absorbed H2O molecule also provides an H-O-H bending vibration that generates absorption peak at 1635 cm-1. The absorption peak at the 794 cm-1 comes from the stretching vibration of the Si-O-Si group, while the absorption peak at 450 cm-1 comes from the bending vibration of the Si-O-Si group.12 The FTIR spectra of MCM-41 BK (before calcination) has some absorption peaks at 2924 cm-1 and 2854 cm-1, which refer to the asymmetric and symmetric vibrations of the CH2 group. The absorption peak at 1450-1500 cm-1 indicates the existence of the cutting vibration of CH2 as well as the asymmetric bending vibration of CH3-N+. Thesee absorption peaks were disappeared on FTIR spectra of MCM-41 AK (after calcination). This result indicates that the CTAB had been removed in the process of calcination.
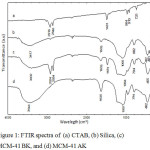 |
Figure 1: FTIR spectra of (a) CTAB, (b) Silica, (c) MCM-41 BK, and (d) MCM-41 AK |
Diffractogram of MCM-41 is presented in Fig. 2. The difractogram of MCM-41 AK has a diffraction peak at the 2θ = 2-3°C which is characteristic for the [100] plane of the hexagonal structure of MCM-41.4-6 However, this diffraction peak did not observed in the diffractogram of MCM-41 BK. This phenomenon was caused by the existence of CTAB in the MCM-41 BK. The CTAB covered the pore of MCM-41, so the hexagonal structure of MCM-41 was not formed yet. In other hand, diffraction peaks at 2θ = 4-7°C indicate the existence of a regular ordered structure on a MCM-41.4-6 It means that MCM-41 was successfully synthesized by sonochemical method in this work.
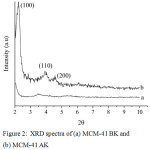 |
Figure 2: XRD spectra of (a) MCM-41 BK and (b) MCM-41 AK
|
The surface morphology of the MCM-41 synthesized in this work was taken by TEM and the TEM micrographs is showed in Fig. 3. The bright part of the TEM image showed that there is no material in the section whereas in the dark indicate the existence of a certain material. The material in this case is the silica wall of the MCM-41. The figure indicates that the pore wall of the MCM-41 is quite regular. The same result was reported some researchers.4 The pore of the MCM-41 is beehive-like structure.
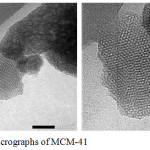 |
Figure 3: TEM micrographs of MCM-41 |
Characterization of K2O/MCM-41 catalyst
The amount of K+ loaded onto the MCM-41 was determined by ICP. Table 2 presents the amount of K+ loaded onto MCM-41. The ICP analysis result indicated that K+ content of K2O(1), K2O(2), K2O(3), and K2O(4)/MCM-41 catalysts was 0.80, 1.35, 1.86, and 2.49 wt.%, respectively. The increasing pattern of K+ content on the catalysts confirmed that the preparation of catalysts were successfully carried out in this work.
Table 2: Amount of K+ loaded onto MCM-41
| Catlysts | K+ content (wt.%) |
| MCM-41 | – |
| K2O(1)/MCM-41 | 0.80 |
| K2O(2)/MCM-41 | 1.35 |
| K2O(3)/MCM-41 | 1.86 |
| K2O(4)/MCM-41 | 2.49 |
Table 3 showed the surface parameters of MCM-41 and K2O(4)/MCM-41 catalyst. After the loading of K+, the specific surface area of MCM-41 was reduced up to 70%. Similar result was observed on their total pore volume values. After the loading of K+, the total pore volume of MCM-41 was reduced up to 65%. This facts make sense if the pore of MCM-41 was filled with K+ and this phenomenon decreased the pore volume and specific surface area of the MCM-41.
Table 3: Surface parameters of MCM-41 and K2O(4)/MCM-41.
|
Sample |
SBET(a) (m2/g) |
Vtotal (b) (cm3/g) |
Pore diameter (Å) |
|
MCM-41 |
1282.33 |
0.84 |
30.49 |
|
K2O(4)/MCM-41 |
253.81 |
0.29 |
30.12 |
note: a) Spesific surface area, b) Total pore volume
Fig. 4 presents the adsorption-desorption isotherm of N2 gas on the MCM-41 and K2O(4)/MCM-41 surface. These adsorption-desorption isotherm pattern were indicated as type IV, which is characteristic for mesoporous material.10 Hysteresis loop that occurred at P/P0 = 0.4 leads to the formation of multilayer of N2 gas on the materials surface.
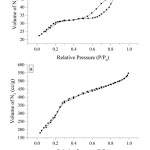 |
Figure 4: Adsorption-desorpstion isotherm of N2 on (a) MCM-41 and (b) K2O(4)/MCM-41
|
Effect of K+content towards biodiesel yield
Prior to the transesterification of used cooking oil, first analysis of free fatty acid content (% FFA) of used cooking oil was carried out. The content of free fatty acids in the cooking oil was very low (0.1881%). So that the transesterification can be done without esterification process.5 The results of transesterification of used cooking oil using K2O(1), K2O(2), K2O(3), and K2O(4)/MCM-41 catalysts at 50, 60, and 70°C were showed in Table 4. The highest yield of biodiesel (79.80 wt.%) was obtained by using K2O(4)/MCM-41 catalyst at 70°C and the lowest yield of biodiesel (30.21 wt.%) was performed by using K2O(1)/MCM-41 catalyst at 50°C. The increasing in the yield of biodiesel was directly proportional to the increasing of K+ concentration at each applied temperature. The increase in biodiesel yield obtained by using the K2O(4)/MCM-41 catalyst shows the most constant increasing than the other catalysts.
|
Catalyst |
Temperature (℃) | Yield (wt.%) |
| K2O(1)/MCM-41 |
50 |
30.21 |
| 60 | 45.48 | |
| 70 | 48.73 | |
| K2O(2)/MCM-41 | 50 | 60.79 |
| 60 | 60.13 | |
| 70 | 65.76 | |
| K2O(3)//MCM-41 | 50 | 66.40 |
| 60 | 75.10 | |
| 70 | 75.45 | |
| K2O(4)//MCM-41 | 50 | 73.31 |
| 60 | 76.62 | |
| 70 |
79.80 |
Fig. 5 presents the results of the biodiesel yield obtained using K2O(1), K2O(2), K2O(3), and K2O(4)/MCM-41 catalysts. The increasing of K+ content in the MCM-41 based catalyst raised the nucleophilic properties of methanol (CH3OH) by converting it from neutral nucleophile into negative charge so it can react with oil to break the used cooking oil into methyl esters and glycerol.
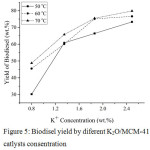 |
Figure 5: Biodisel yield by diferent K2O/MCM-41 catlysts consentration.
|
Effect of transesterification temperature toward biodiesel yield
The transesterification results obtained at 50, 60, and 70°C using all catalysts were showed in Fig. 6. The optimum temperature of transesterification of used cooking oil using K2O(1), K2O(2), K2O(3), and K2O(4)/MCM-41 catalysts to produce the highest biodiesel was 70°C. The highest biodiesel yield was about 79.80 wt.% obtained by using K2O(4)/MCM-41 catalyst at 70°C. The above explanation lead to the fact that the K2O(4)/MCM-41 catalysts had a greater activity in transesterification of used cooking oil than the others catalysts and its catalytic activity was maximum at 70°C.
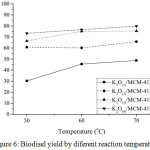 |
Figure 6: Biodisel yield by diferent reaction temperature |
Liu and Lampert13 concluded that the process of transesterification of used cooking oils using methanol will more quickly when the temperature is raised near the boiling point of methanol. By the heating of the oil molecules, they will be dispersed and distributed into the methanol molecule and then reacts, so the glyceride bond will breaked to form methyl esters with K2O/MCM-41 catalyst. The heating process increases the movement of molecules of oil-methanol mixture resulting in collisions between molecules and providing sufficient energy to reach the activation complex and transesterifkasi reactions14. Low yields obtained at 50°C were mainly caused by the low possibility esterification of free fatty acid.
Determination of Catalyst lifetime in Transesterification Reactions
Table 5: Biodiesel yield from transesterification of used cooking oil using K2O(4)/MCM-41
|
Run |
Yield (wt.%) |
|
1st |
79.80 |
|
2nd |
60.35 |
|
3rd |
56.84 |
The transesterification of used cooking oil using K2O(4)/MCM-41 catalyst at 70°C was carried out within three times for determination of the catalyst lifetime. Table 5 shows yield of biodiesel obtained from transesterification of used cooking oil using K2O(4)/MCM-41 catalyst for three times. The results indicated a significant decreasing in the activity of K2O(4)/MCM-41 catalyst on the second and the third run. Fig. 7 shows plotting graph of reaction time vs yield of biodiesel. The catalyst lifetime was determined using regression linear formula obtained from the graph.5 The linear regression is y = -5.74x + 88.62. By using the regression linear formula, the lifetime of the K2O(4)/MCM-41 catalyst is about 15.41 h. it means that transesterification of used cooking oil using K2O(4)/MCM-41 at 70°C will not produce biodiesel after 15.41 h.
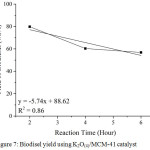 |
Figure 7: Biodisel yield using K2O(4)/MCM-41 catalyst.
|
Conclusion
Synthesis and characterization of MCM-41 and K2O/MCM-41 from Lapindo mud by sonochemical method for transesterification of used cooking oil to produce biodiesel had been conducted. The diffractogram of MCM-41 had a characteristic peak of MCM-41 at 2θ = 2-3°C. The TEM micrographs of MCM-41 shows an ordered pore distribution with a hexagonal shape. The MCM-41 and K2O(4)/MCM-41 have spesific surface area of 1282.33 and 225.81 m2/g and average pore diameter of 30.49 and 30.12 Å, respectively. The ICP analysis result shows that potasium content in K2O(1), K2O(2), K2O(3), and K2O(4)/MCM-41 catalysts was 0.80, 1.35, 1.86, and 2.49 wt.%, respectively. The highest yield of biodiesel was about 79.80 wt.% and it was obtained at 70 ℃ using the K2O(4)/MCM-41 catalyst. Catalyst lifetime of the K2O(4)/MCM-41 for the transesterification of used cooking oil was about 15.41 h.
Acknowledgements
This work was conducted under research grant of PUPT 2017 Gadjah Mada Univercity. The authors thank The Indonesian Ministry of Research, Technology, and Higher Education for financial support (Contract number: 2351/UN1.P.III/DIT-LIT/LT/2017).
We gratefully acknowledge the funding from USAID through the SERA program – Centre for Development of Sustainable Region (CDSR).
References
- Wijaya, K.; Herlina, I.; Trisunaryanti, W.; Nuryono; Simbolon, S. Asian J. Chem. 2018, 30, 724–730.
CrossRef - Abdulloh, A.; Aminah, N. S.; Triyono; Mudasir; Trisunaryanti, W. AIP Conf. Proc. 2016, 1718, 070001-2-070001-10.
CrossRef - Wijaya, K.; Baobalabuana, G.; Trisunaryanti, W.; Syoufian, A. Asian J. Chem. 2013, 25, 8981–8986.
- Ifah, A. Al; Trisunaryanti, W.; Triyono; Dewi, K. Int. J. ChemTech Res. 2016, 9, 382–387.
- Mahardika, I. B. P.; Trisunaryanti, W.; Triyono, T.; Wijaya, D. P.; Dewi, K. Indones. J. Chem. 2017, 17, 509–515.
CrossRef - Triyono; Trisunaryanti, W.; Putri, A. D. W. I.; Lutfiana, A.; Dewi, K. Asian J. Chem. 2018, 30, 953–957.
CrossRef - Mubarok, M. A. S. Al; Setiawan, L. P.; Utami, M.; Trisunaryanti, W. Int. J. Acad. Sci. Res. 2014, 2, 31–36.
- Kusumastuti, H.; Trisunaryanti, W.; Falah, I. I.; Marsuki, M. F. Rasayan J. Chem. 2018, 11, 522–530.
CrossRef - Trisunaryanti, W.; Lisna, P. S.; Kartini, I.; Sutarno; Falah, I. I.; Triyono Asian J. Chem. 2016, 28, 996–1000.
CrossRef - Marsuki, M. F.; Trisunaryanti, W.; Falah, I. I.; Wijaya, K. Orient J Chem 2018, 34, 955–962.
- Nurmalasari; Trisunaryanti, W.; Sutarno; Falah, I. I. Int. J. ChemTech Res. 2016, 9, 607–614.
- Masykuroh, A.; Trisunaryanti, W.; Falah, I. I.; Sutarno Int. J. ChemTech Res. 2016, 9, 598–606.
- Liu, L.; Lampert, D. J. Am. Oil Chem. Soc. 1999, 7, 783–787.
CrossRef - Noureddini, H.; Zhu, D. J. Am. Oil Chem. Soc. 1997, 74, 1457–1463.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









