Adsorption Process of Cu(II) cations by using the Waste of Phosphate Rock (WPR)as novel adsorbent material
Hind Argani , Mona Latifa Bouamrani, Samia Yousfi, M. Hammed El-Kouali, Mohammed Talbi and Rachid Atmani
, Mona Latifa Bouamrani, Samia Yousfi, M. Hammed El-Kouali, Mohammed Talbi and Rachid Atmani
Research Laboratory of Analytical Chemistry and Physical Chemistry of Materials, Sciences Faculty Ben M'sik, University Hassan II - Casablanca, Morocco.
Corresponding Author E-mail: arganihind@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/3404026
Article Received on : 21-01-2018
Article Accepted on : 07-05-2018
Article Published : 26 Aug 2018
The capacity of using WPR as an unused future adsorbent for the elimination of copper ions from synthetic watery solution was carried out in batch mode. The adsorption ability of Cu2+ by WPR was estimated in accordance with: initial concentration, time of contact, temperature and pH. The temperature rise resulted a higher adsorption of Copper ions by the waste of phosphate rock (WPR). The data collected was tested by using Langmuir and Freundlich isotherms. The non-linear regression procedure was the way to obtain the parameters of adsorption isotherms. The errors analysis was finalized to find out if the model was best to adapt with the experimental protocol. In the light of the obtained results and the various previous researches carried out and published in this sense, the different conclusions were proposed about the adsorption mechanism. The thermodynamic parameters were estimated. The ΔH of adsorption was positive so the process is endothermic and ΔG was negative Therefore the adsorption was spontaneous.
KEYWORDS:Adsorption; Copper Ion; Heavy Metals Removal; Phosphate Rock; Thermodynamic Parameters
Download this article as:| Copy the following to cite this article: Argani H, Bouamrani M. L, Yousfi S, El-Kouali M. H, Talbi M, Atmani R. Adsorption Process of Cu(II) cations by using the Waste of Phosphate Rock (WPR)as novel adsorbent material. Orient J Chem 2018;34(4). |
| Copy the following to cite this URL: Argani H, Bouamrani M. L, Yousfi S, El-Kouali M. H, Talbi M, Atmani R. Adsorption Process of Cu(II) cations by using the Waste of Phosphate Rock (WPR)as novel adsorbent material. Orient J Chem 2018;34(4). Available from: http://www.orientjchem.org/?p=48756 |
Introduction
The increase in industrial activity influences potential toxic pollution by heavy metals, which has increased vastly over the last decennium. The enormous amount of waste (solid, liquid and gaseous) generated by industrial activities could only increase the penetration of heavy metals into the environment, especially water, being the major target of this scourge because of its massive use in many functions or industrial applications, this gradually leads to water degradation states andendangers human health. Removing poisonous heavy metals or reducing their concentrations to the authorized limits that have been fixed in the legislation before discharge is thus responsible, especially with the rise in industrial sector. Among the most dangerous pollutants is Cu(II) copper. Copper pollution in water comes mainly from the following sources: boilermaking used in the majority of industrial sectors, manufacturing of thermal transfer equipment, feedingstuffs as dietary supplement, vegetable and fruit growing as afungicide, treatment of phytosanitary products. etc.
Several treatment technologies are applied for the elimination of poisonous metals from the aqueous solution, such as ion exchange, reverse osmosis, adsorption, complexation and precipitation.1 Recently, many studies have recognized great prominence of ion(s) exchange properties of the apatites and their application in different fields.2 Also, several authors have demonstrated the potential of calcined phosphate (CP) as a basic or acid heterogeneous catalyst for for various chemical reactions.3,4,5
The increase in industrial activity influences potential toxic pollution by heavy metals, which has increased vastly over the last decennium. The enormous amount of waste (solid, liquid and gaseous) generated by industrial activities could only increase the penetration of heavy metals into the environment, especially water, being the major target of this scourge because of its massive use in many functions or industrial applications, this gradually leads to water degradation states and endangers human health. Removing poisonous heavy metals or reducing their concentrations to the authorized limits that have been fixed in the legislation before discharge is thus responsible, especially with the rise in industrial sector. Among the most dangerous pollutants is Cu(II) copper. Copper pollution in water comes mainly from the following sources: boilermaking used in the majority of industrial sectors, manufacturing of thermal transfer equipment, feedingstuffs as dietary supplement, vegetable and fruit growing as afungicide, treatment of phytosanitary products. etc.
The present study focuses on the copper removal from aqueous solutions by using the adsorption method onto the fine fraction of phosphate rock. The effects of possible competitions of bivalent ions present in industrial effluents are not considered inthis study. The estimation of findings has been determined by using Langmuir and Freundlich equations. Furthermore, the effect of pH and initial metal concentrations on sorption efficiency are investigated. The thermodynamic parameters are estimated.
Materials and Methods
Preparation of Phosphate and characterization
The phosphate mineral employed in this research is obtained from the region of Khouribga in Morocco. PR was well homogenized, only the range of particle size less than 180 µm was used. This fraction was cleaned with distiller water then cured in oven at 105°C for 24h.
The IR spectrophotometer was used to identify the surface functional groups. Pellet of analysis was prepared by mixing 2% of PR in KBr. Additionally, X-ray diffraction was applied to characterize the crystalline phases, using CuKa radiation. Ultimately the lattice parameters were obtained using the Software “Camail”.
Experimental Procedure
Stock Copper solutions were prepared by dissolving 1000 mg of copper sulphate pentahydrate [CuSO4.5H2O] metal salt into 100 ml of distilled water. it was chosen for his easy solubility in water. It is worth mentioning that the [CuSO4.5H2O] metal salt was preserved with concentrated HNO3. Batch adsorption was conducted. In 250 ml Erlenmeyer containing different amounts of phosphate rock as adsorbent and 100 ml of Copper solutions containing 200mg L−1 .The solution pH was adjusted to 5 with tampon solution at the desired temperatures (298, 303, 313°K) to assess the influence of the temperature on the adsorption process. The Mixtures were agitated until equilibrium was reached. The suspensions were stirred at a constant agitation rate of 400 rpm for different time intervals than the solid (PR) was separated from the mixture through a 0.45 mm membrane filter (MFS). The metal concentrations in the filtrate were determined by complexometric titration with EDTA (disodium salt) in the presence of murexide as an indicator. the kinetic study was investigated by 0,1g of sorbent suspended in 100mL of different initial Cu2+ solutions (100, 200, 400 mg.L-1) and the initial pH was maintained at 5. The effect of initial pH on the adsorption kinetics of metal ion was conducted in a pH range of 2–6 with a constant solid phosphate weight (0,2g) suspended in 100 ml Cu(II) solutions with a concentration of 100mg/L. The initial pH of the solutions has been adjusted through 0.1M HCl and 0.1M NaOH. The mixture was stirred for the appropriate time. The adsorption of Cu(II) adsorbed onto PR at time t(min),qt(mg/g)was determined using the following equations:
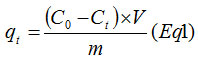
where, C0 and Ct are respectively the initial Cu(II) concentration (mg/L) and, the supernatant Cu(II) concentration at time t (mg/L), m is the weight of PR(g), and V is the volume of the solution (L).
Results and Discussion
Characterization of the Adsorbent
The analysis of NP by IR adsorption spectrum (Fig.1) shows several bands, especially those attributed to PO43- groups and carbonate ions CO32-. Indeed, the absorption bands of PO43- ions are characterized by two adsorption domains located between 1100 and 900 cm-1 and between 500 and 600 cm-1. The first corresponds to symmetric and antisymmetric vibrations of PO (ν1 + ν3) and the second is related to the vibrations of deformation of O-P-O (ν2 + ν4). The bands at 1458, 1430 and 870 cm-1 correspond to carbonate groups. A wide band located at 3650 cm-1 represents vibrations of hydroxyl groups O–H. The phosphate sample shows two bands of low intensity at 2850 cm-1 and 2875 cm-1, which may correlate with C–H stretching. The adsorption peak at 450 cm-1 and 725 cm-1 can be associated to Si-O rocking.
The RX diffractogram demonstrated that the solid is crystallized in the hexagonal system of space group P63/m. It was shown that the sample of NP consists of a carbonated fluorapatite with other secondary phases, which are attributed to quartz and dolomite.
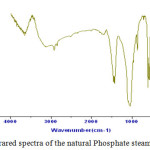 |
Figure 1: Infrared spectra of the natural Phosphate steamed at 100°C.
|
Table:1 Lattice parameters of NP compared to those of fluorapatite, francolite and synthetic hydroxyapatite.
|
Parameters (Å) |
NP (this study) |
Fluora-patite6 |
Froncolite6 |
Hydro-xyapatite7 |
|
a |
9.238 |
9.372 |
≤9.360 (variable with the content of CO2 and H2O) |
9.432 |
|
c |
6.405 |
6.888 |
≤ 6.890 (variable with H2O) |
6.882 |
The lattice parameters a and c of the NP study are presented in Table 1.
The IR absorption spectrum (Fig.2) shows carbonate fluorapatite peaks while the lattice parameters obtained allow to consider that it is a francolite. Some of the carbonate apatites in Sedimentary phosphate rocks have a lower F content than stoichiometric fluorapatite and may contain significant amounts of hydroxyl in their structures. However, they can meet the definition of Francolites (significant CO2 with more than 1 percent of F).8 They have crystallographic, chemical and physical properties that differ significantly from those of Francolite that contain excess fluorine, This is suggested by (Van Kauwenbergh And McClellan 1990-1995) and cited by Zapata and Roy.8
Effect of pH
The pH is an important factor. For the real application of PR in the adsorption process of metal ions from aqueous solutions. It acts very strongly not only on the surface load of the adsorbent, but also on the speciation of metals in water, i.e. on the proportions of their different chemical forms.
The metallic cations in aqueous solution can be converted into different chemical forms by the effect of pH change. Fig.2 shows an example of the chemical speciation of copper, as a function of pH (the main species are represented).9
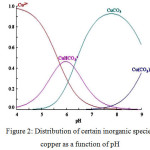 |
Figure 2: Distribution of certain inorganic species |
The sorption capacity of PR to remove Copper ions from aqueous solutions was examined for various pH values. The pH of the different solutions has been corrected from 2 to 6. These values were chosen to prevent deposition of metals at greater pH values. The figure 3 showed that, the pH of the solution influences copper adsorption.
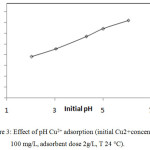 |
Figure 3: Effect of pH Cu2+ adsorption (initial Cu2+ concentration 100 mg/L, adsorbent dose 2g/L, T 24°C) |
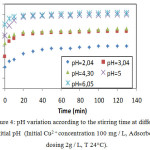 |
Figure 4: pH variation according to the stirring time at different initial pH (Initial Cu2 + concentration 100 mg / L, Adsorbent dosing 2g / L, T 24°C). |
It can be seen that the uptake of copper increased with an augmentation of pH values. The maximum adsorption capacity for copper was found to be at pH value between 4 and 6 while it was insignificant under a pH3.
The most appropriate pH values for a maximum removal of copper (II) were found to be 5-6. The equilibrium adsorption capacity of the metal ion considered at these pHs was 29,77mg g−1. This increase in copper removal by raising the pH was also indicated. for the jordanian low- grade phosphate rock and calcined phosphate.10,2
Indeed the pH value of aqueous solution may affect the surface charge of adsorbent.11 Sarioglu, Atay and Cebci12 have suggested that below pH 3, the low adsorption capacity of copper can be explained by the repulsive electrostatic forces that occur between phosphate particles and copper ions and to the competition effects with ion H3O+. This hypothesis was also suggested by Aklil, Mouflih, and Sebti.2 Therefore, by adopting this assumption, it can be readily explained that the increase in copper removal at a pH values beyond 3 , was due to the decrease of the competition of the ions H3O+. This increase in copper removal by increasing pH can be attributed evenly to the preferential adsorption of the hydrolysed species (CuOH+).It may be also explicited by the increase of adsorption sites related to the pH such as the carboxyl groups of organic matter, hydroxyl groups of oxides and clay minerals, since the fine fraction of the natural phosphate used as adsorbent in this study contains essentially the argillous components “Exo-gangue”. Most of metal ions precipitate as hydroxides, thus pH effect has not been evaluated for elevated pH values. Sugiyama and al., 2001 proposed two mechanisms for the capacity of hydroxyapatite to embarrass divalent cations: the first is through an ion-ion exchange mechanism and the second is through the dissolution of mineral apatites and the precipitation or coprecipitation. Smičiklas and al13 have reported in their article that the HAP solubility diminishes with the rise of pH of the solution, thereby the phosphate solubility assumptions appears to be not usable by increasing the pH in the case of this study, thus consequently, the mechanism for ion-exchange of copper (II) by PR may not unfold via a dissolution-precipitation mechanism.2
Aklil, Mouflih, and Sebti suggested that the reaction of CP with Cu2+ at pH 2 and 3 can result in the formation of a solid-solution-type apatite like Ca10−xCux(PO4)6F2. Similarly, we suggested at pH2 and pH3, that the mechanism for copper-exchange may proceed by formation of solid-solution. On the other hand, Smičiklas and al, are also proposing mechanisms that control and predominate the adsorption of cations at high pH such as precipitation of insoluble hydroxides or the existence of electrostatic forces occurring at the liquid solid interface.13 This means that the adsorption of free Cu2+species is not the only phenomenon responsible for the immobilization of these heavy metals.
It can be noticed from fig. 4 that following the retention of copper there is always an increase in the pH of the solution, in fact the dissolution of the NP in water can be given by the equation:
Ca10(PO4)6F2 + 12H2O → 10Ca2+ + 6H2PO4– + 2F– + 12OH– (Natural Phosphate) (Water) (Dissociation products)
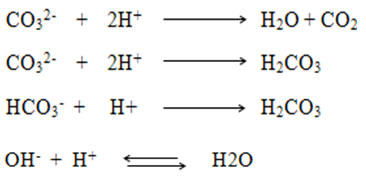
This dissolution reaction is more favored at lower pH values. Although the above reaction is for PR type fluorapatite, it is applicable to other mineral apatites group, of which reactive phosphate (RP) (8). As shown in the reaction mentioned above , the dissolution of the NP gives rise to the release of hydroxyl ions into the solution. The neutralization of hydroxyl ions generated by the acidity of the aqueous medium, allows the process of dissolution of the PR to continue14 until total dissolution of the phosphate and the complete neutralization of the medium gives rise to an equilibrium state of pH (fig.3). In fact, the neutralization of the medium may also be due to the carbonate or bicarbonate ions released by the surface according to the reactions respectively represented below:
Or by ionic product of water according to the following reaction:
Effect of Initial Copper Concentration
The experimental results of sorption at distinct initial concentrations of copper on natural phosphate are shown in Figure 5.
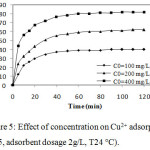 |
Figure 5: Effect of concentration on Cu2+ adsorption (pH5, adsorbent dosage 2g/L, T24°C).
|
The uptake of Cu2+ was found to be reliant on the initial concentration, the sorption capacity of copper increases with the increase of contact time. The amount of copper adsorbed increases with the increase of initial Cu2+concentration at the range of experimental concentration. The adsorption is fast at an initial stage, then becomes no observable at a second stage and arrives at the equilibrium in about 90 min. At high concentration (400 mg/L) the active sites of NP were besieged by much more Cu2+ ions. Copper removal decreased with the increase in initial copper concentrations. This is attributed to an insufficiency in the number of sorption sites at higher concentrations.
Adsorption Isotherms
Not only does the adsorption isotherm reveals the adsorption capacity of the adsorbent, but it also describes the interaction mechanism between the adsorbate and the adsorbent.15 Two commonly used isotherms, Langmuir16 and Freundlich17 were involved in in this paper. The nonlinear Langmuir and Freundlich isotherms are expressed as:
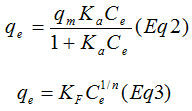
Where Kf and n are the Freundlich constants, they respectively provide information on the adsorption capacity and adsorption intensity.18 Ce is the equilibrium concentration (mg L−1); qe is the amount of Cu2+ ion adsorbed at equilibrium (mg/g); qm represent the monolayer adsorption capacity and Ka is the Langmuir constant related to the energy of adsorption. A nonlinear plot of Langmuir and Freundlich equilibrium equations 2 and 3 respectively were employed to determine the value of isotherm parameters (table 2). In order to evaluate the ability of Langmuir and Freundlich isotherms to establish a correlation with experimental results, the fitted plots from each isotherm were shown with the experimental data for the copper uptake onto PR at the temperatures 298, 303 and 313K in Fig.6 and
Fig.7. The results were further analyzed using two error functions, the Sum of the Squares of the Errors (SSE) and the Average Relative Error (ARE). Their calculated expressions are as following19 :
The sum of the squares of the errors (SSE):
![]()
The average relative error (ARE):
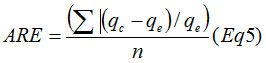
Where n is the number of experimental data points, qc is the predicted (calculated) data according to the models and qe is the experimental data.
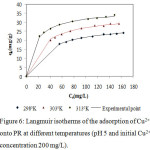 |
Figure 6: Langmuir isotherms of the adsorption of Cu2+onto PR at different temperatures (pH 5 and initial Cu2+concentration 200 mg/L).
|
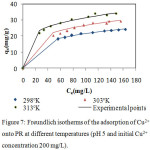 |
Figure 7: Freundlich isotherms of the adsorption of Cu2+onto PR at different temperatures (pH 5 and initial Cu2+concentration 200 mg/L).
|
Table.2: Isotherm parameters for Langmuir and Freundlich models at different temperatures
|
Temperature (K) |
Freundlich | ||||
| KFL/g | n | R² | SSE | ARE | |
| 298 | 6,318 | 3,7 | 0,9993 | 0,580 | 0,0107 |
| 303 | 8,237 | 3,9 | 0,9948 | 8,841 | 0,0354 |
| Temperature (K) | Langmuir | ||||
| qmmg/g | KL L/mg | R² | SSE | ARE | |
| 298 | 29,30 | 0,0283 | 0,9998 | 1,156 | 0,0054 |
| 303 | 34,32 | 0,0371 | 0,9954 | 1,559 | 0,0144 |
| 313 | 36,72 | 0,0645 | 0,9967 | 1,065 |
0,0085 |
The nonlinear regressive “method of least squares” is used to determine the isotherm parameters. The model parameters were calculated by minimizing the error function over the concentration range studied..It can be observed from table 2 that the experimental data fit both the Langmuir and Freundlich isotherm on the temperature range studied. This is concluded from the Correlation coefficient values (R2) which were found to be bigger than 0.99 for Langmuir and Freundlich isotherms. The Langmuir equation is very suitable to the experimental data. Not only according to R² but also according to the values of ESS and ARE. Furthermore, the value of R2 from Langmuir was bigger and the predicted curve obtained from Langmuir model passes nearer to the experimental points (fig.6).Langmuir proposed his theory by making the following assumptions: the adsorbent solid has a limited adsorption capacity (qm), all active sites are identical (uniform energy), and they can complex only one solute molecule (monolayer adsorption) and the interactions between adsorbed molecules are null.20
The application of these no linearized forms of the equations of Langmuir and Freundlich in the case of this study made it possible to verify that these two models were perfectly applicable and that the adsorbent capacity varies in the same direction with the two models. Indeed the surface of the PR is necessarily heterogeneous, thus all adsorption sites are not energetically homogeneous, so if the experimental data fit well the Langmuir isotherms, it is likely that this agreement is purely fortuitous.
Other studies have shown that the Langmuir isotherm was appropriate for the description of data for copper adsorption onto PR2 As shown in Table 2, the adsorption amount of the metal ions and all the parameters of isotherms have all increased with increasing the temperature. Consequently the temperature has a positive influence on the adsorption capacity. In recent studies, it has been found that desorption of metal ions retainedby apatite with a variety of solvents is not significant.21 This affirms the chemisorption nature of the process. The monolayer adsorption capacities of WPR (qm), obtained from Langmuir equation were 29.30, 34.32 and 36.72 mg/g at 298, 303, 313K, respectively. These findings encourage the use of phosphate waste in reducing heavy metals which may accumulate in the environment and are toxic to plants, humans and animals. The large reserve of natural phosphate, the abundant quantity of this ore, and fine waste generated by the process of mining and concentration, its valuable characteristics and the findings obtained in the present study suggests the fine fraction of natural phosphate as a new concurrent of the other celebrated adsorbents for wastewater treatment. In the Freundlich model all values of KF showed the easy uptake of copper ions with high adsorption capacity of WPR which has varied with temperature. The values of n were found between 1 and 10 and indicated favorable adsorption of copper (II) on the PR at all temperatures studied.22
The most abundant ligand is water. Thus, each metal cation is surrounded by a procession of water molecules, in the general number of 6 which corresponds to the sphere of hydration of the cation.25 Thus, the copper present in the aqueous solution in its ionized form (Cu2+), accompanied by its sphere of hydration (Cu (H2O) 62+).
The proximity of the positive charge of the cation to that of the protons has the effect of deprotonation of the water molecules surrounding the cation. But the hydrolysis is significantly dependent on the pH of the solution and it is specific to ions.25 Major inorganic ligands such as SO4-2, NO3– react very weakly with copper in solution. On the other hand, the major ion Cl– is known to form relatively stable complexes with copper.
These inorganic ligands compete with functional groups of organic matter for which copper has a very high affinity.26,27 The metals can also be complexed by the substances composing the OM (Organic Matter) and more particularly by the carboxylic sites (groups contributing to the majority of complexation sites). Copper is known to preferentially bind to carboxyl groups.9
The figure 7 illustrates the interactions between the free copper (Cu2 +) ion and the ligands with which complexes can form.
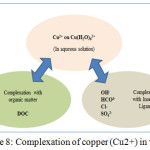 |
Figure 8: Complexation of copper (Cu2+) in water
|
The sorption of copper ions on phosphate rock is done due to its mineral or organic surface. Thus during the sorption on the PN, the copper can be:
Included in apatite crystal lattices and reactive secondary constituents of PN (e.g. : iron and magnesium oxide) by cation exchange with equivalent charge cations (e.g.: formation of solid solutionCa10−xCux(PO4)6F2)
Simply introduced by diffusion inside the solid to fill a vacuum or replace an atom of the solid.
Chelated with organic compounds molecules.
Complexed with an anionic molecule of the surface by the formation of a covalent chemical bond. In this case, the copper cation surrounded by its hydration sphere can be formed of a complex called external sphere (see Figure 8), which is less stable and involves electrostatic bonds, and therefore results in neutralization of the charge of the solid (non-specific adsorption). Or to form a more stable internal sphere complex by stronger bonding forces neutralize the charge of the solid (non-specific adsorption), or to form a more stable internal sphere complex by stronger bonding forces, i.e. involves covalent bonds or certain combinations of ionic bonds after which, the adsorbed copper cation can be considered part of the solid and reverse the intrinsic charge of the surface (specific adsorption or chemisorption).25,28
Precipitated on the surface of the NP mainly in the form of hydroxide, carbonates, phosphates or sulphides.
Furthermore, the isotherm observed for the sorption of copper ion on our adsorbent was of type L subtype 2 in the classification of Giles et al (1960). Subgroup 2 indicates that the theoretical monolayer has been finalized. (29) .According to Echeverria (1998) etCao (2004), the shape of isotherm obtained reflects also surface adsorption or complexation mechanism).29,30
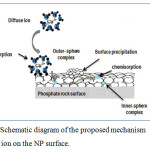 |
Figure 9: Schematic diagram of the proposed mechanism of copper ion on the NP surface.
|
Estimation of thermodynamic parameters
With the aim of evaluate the effect of temperature on the adsorption of Cu2+ on phosphate rock, thermodynamic parameters such as the free energy change DG◦((kJ.mol−1), enthalpy change DH◦(kJ.mol-1), and entropy change DS◦(J.mol−1. K−1) were determined, using the following equations: (Van’t Hoff equation)
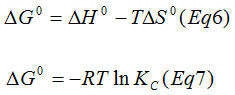
Were T is the absolute temperature in Kelvin, R is the gas constant (8.314×10−3), and Kc is the apparent equilibrium constant and is defined as31:
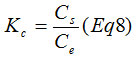
Where Cs is the concentration of Copper ions on the adsorbent at equilibrium (mg.l−1).The change of enthalpy (DH◦) and entropy (DS◦) can be estimated from the slope and intercept of a van’t Hoff equation of DG◦ versus T. The thermodynamic parameters are listed in Table. The negative DG° values 2.350, 7.894 kJ/mol at 25, 30 and 40°C, respectively, declines with a rise in temperature and indicates the spontaneous nature with a high preference of the copper ions adsorption onto PR. A high temperature favored the adsorption. The positive calculated DH° value 293.61 kJ/mol, confirmed the endothermic nature of Cu2+adsorption. Whereas the positive value of DS°(993,91 J/mol.K) reflects the Raising of the randomity at the solid-solute interface during adsorption.19 The value of enthalpy change(DH°) was between 84 and 420kJ/mol32 indicating endothermic and chemisorption of the adsorption process. Chemisorption usually increases with temperature. Thereby a rise of temperature can often cause physical adsorption to change to Chemisorption.
Table 3: Thermodynamic parameters of adsorption of Cu2+onto PR.
| Temperature °K | DG° Kj/mol | DH° Kj/mol | DS° J/mol |
| 298 | -2.35 | 293.61 | 993,91 |
| 303 | -7.89 | ||
| 313 | -17.37 |
Conclusion
The main of this research was to evaluate the copper ions uptake capacity of the fine fraction of natural phosphate, as an abundant industrial waste. The adsorption of the copper ion relies on the pH of the solutions. The maximum adsorption is observed between pH 4 and 6. The Data generated from sorption isotherms at several temperatures. are better fitted by Langmuir model. The retention of copper (II) ions on WPR is favoured at higher temperature values. The thermodynamics study confirm that the adsorption occurs spontaneously and it is an endothermic process. The enthalpy value indicating the chemisorption nature of copper adsorption onto WPR. The results show that WPR is an efficient novel adsorbent for copper ionselimination. , so it should be possible to test his effectiveness to trap other metal ions.
References
- Ngah, WS W.; Endud, C. S. and Mayanar, R.; “Removal of Copper (II) Ions from Aqueous Solution onto Chitosan and Cross-Linked Chitosan Beads”; Reactive and Functional Polymers. 2002, 50 (2), 181–190.
CrossRef - Aklil, A.; Mouflih, M.; and Sebti, S.; “Removal of Heavy Metal Ions from Water by Using Calcined Phosphate as a New Adsorbent”; Journal of Hazardous Materials. 2004, 112 (3), 183–90.
CrossRef - Macquarrie, D. J.; Nazih, R. and Sebti, S.; “KF/Natural Phosphate as an Efficient Catalyst for Synthesis of 2′-Hydroxychalcones and Flavanones”; Green Chemistry.2002, 4 (1), 56–59.
CrossRef - Sebti, S.; Rhihil, A.; and Saber, A.; “Heterogeneous Catalysis of the Friedel-Crafts Alkylation by Doped Natural Phosphate and Tricalcium Phosphate”; Chimestry Letters. 1996, 8.
- Fraile, José, M.; García, José I.; José A. Mayoral, Sebti, S. and Tahir, R.; “Modified Natural Phosphates: Easily Accessible Basic Catalysts for the Epoxidation of Electron-Deficient Alkenes”; Green Chemistry. 2001, 3 (6), 271–274.
CrossRef - Elliott J.C.; Structure and Chemistry of the Apatites and Other Calcium Orthophosphates-Elsevier Science .1994.
- Kay, M.I.; Young, R.A.; and Posner, A.S;“Cristal Structure of Hydroxyapatite”. 1964, 204, 1050–1052.
- Zapata, F. and Roy, R.N.; “Utilisation des phosphates naturels pour une agriculture durable” ; la Division de la mise en valeur des terres et des eaux de la FAO et de l’Agence internationale de l’énergie atomique Edition. 2004.
- LE GOFF, F. and BONNOMET, V.; “Devenir et comportement des métaux dans l’eau : biodisponibilité et modèles BLM”; Ministère de l’Ecologie et du Développement Durable Direction de l’Eau à Paris, Convention DE n° CV03000081. 2004.
- MuntherIssa, K.; “The Potential Use of Low-Grade Phosphate Rocks as Adsorbent”; Chemical Engineering & Technology. 2002,25 (9), 921–924.
CrossRef - Yu, L.; and Yong-ming L.; “The Adsorption Mechanism of Anionic and Cationic Dyes by Jerusalem Artichoke Stalk-Based Mesoporous Activated Carbon”; Journal of Environmental Chemical Engineering. 2014, 2(1), 220–29.
CrossRef - Sarioglu, M.; Atay, A.; Cebci, Y.; Removal of copper from aqueous solutions by phosphate rock, Desalination.2005, 181,303-311.
CrossRef
- Smičiklas, I., Onjia, A.; Raičević, S.; Janaćković, đ. and Mitrić, M.; “Factors Influencing the Removal of Divalent Cations by Hydroxyapatite” ; Journal of Hazardous Materials. 2008,152 (2), 876–84.
CrossRef - H.CHIEN, S; “Thermodynamic Considerations on the Solubility of Phosphate Rock”; 1977, 123 (2).
- Ma, J.; Fei ,Y.; Lu ,Z.; Lu J.; Mingxuan, Y.; Jingshuai, L.; Yuhang, T.; Haibo, F.; Zhiwen,Y.; and Junhong C.; “Enhanced Adsorptive Removal of Methyl Orange and Methylene Blue from Aqueous Solution by Alkali-Activated Multiwalled Carbon Nanotubes”; ACS Applied Materials & Interfaces. 2012,4 (11), 5749–60.
CrossRef - Langmuir, I.; “The Constitution and Fundamental Properties of Solids and Liquids”; Journal of the Franklin Institute. 1917,183 (1), 102–105.
CrossRef - Foo, K.Y.; and Hameed, B.H; “Insights into the Modeling of Adsorption Isotherm Systems”; Chemical Engineering Journal .2010,156 (1), 2–10.
CrossRef - Ho, Yuh-S.; and McKay, G. “Sorption of Dye from Aqueous Solution by Peat”; Chemical Engineering Journal. 1998, 70 (2), 115–124.
CrossRef - Han, R.; Yi W.; Weihua Z.; Yuanfeng W. and Jie S; “Comparison of Linear and Nonlinear Analysis in Estimating the Thomas Model Parameters for Methylene Blue Adsorption onto Natural Zeolite in Fixed-Bed Column.”; Journal of Hazardous Materials. 2007,145 (1–2), 331–35.
CrossRef - Yaacoubi, H.; Zidani, O.; Mouflih, M.; Gourai, M.; and Sebti, S; “Removal of Cadmium from Water Using Natural Phosphate as Adsorbent.” Procedia Engineering. 2014, 83, 386–93.
CrossRef - Elouear, Z.; Bouzid, J.; Boujelben, N.; Feki, M.; Jamoussi, F. and Montiel, A.; “Heavy Metal Removal from Aqueous Solutions by Activated Phosphate Rock”; Journal of Hazardous Materials. 2008, 156 (1–3), 412–20.
CrossRef - Aksu, Z.; “Determination of the Equilibrium, Kinetic and Thermodynamic Parameters of the Batch Biosorption of Nickel (II) Ions onto Chlorella Vulgaris”; Process Biochemistry. 2002, 38(1), 89–99.
CrossRef - El Ouardia, E.; “Étude de La Calcination Du Phosphate Clair de Youssoufia (Maroc)” ; Afrique Science: Revue Internationale Des Sciences et Technologie.2008,4 (2).
- Serpaud, B., R; Al-Shukry, M.C.; and Matejka, G.; “Adsorption des métaux lourds (Cu, Zn, Cd et Pb) par les sédiments superficiels d’un cours d’eau: rôle du pH, de la température et de la composition du sédiment” ; Revue des sciences de l’eau. 1994, 7 (4), 343.
- Navel, A.; “Distribution, Spéciation Impact et Transfert Du Cuivre Dans Un Sol Sous Vigne: Rôle de La Structuration Spatiale et Du Statut Organique”; Université de Grenoble. 2011
- Arias, E.; Nóvoa M.; Pateiro, M.and López-Periago E.; “INFLUENCE OF AGING ON COPPER FRACTIONATION IN AN ACID SOIL”; Soil Science. 2007,172 (3), 225–32.
CrossRef - Li, W.; Min Z. and Huairui S.; “Distribution and Fractionation of Copper in Soils of Apple Orchards (5Pp)”; Environmental Science and Pollution Research – International. 2005, 12 (3), 168–72.
CrossRef - Stumm, W.; Laura, S.; and Barbara, S.; Chemistry of the Solid-Water Interface: Processes at the Mineral-Water and Particle-Water Interface in Natural Systems; New York: Wiley.1992
- Echeverria, J. C.; Morera, M. T.; Mazkiaran, C. and Garrido, J. J.; “Competitive Sorption of Heavy Metal by Soils. Isotherms and Fractional Factorial Experiments”; Environmental Pollution.1998, 101 (2), 275–284.
CrossRef - Cao, X.; Lena, Q. M.; Dean, R. R.; and Chip, S. A.; “Mechanisms of Lead, Copper, and Zinc Retention by Phosphate Rock”; Environmental Pollution. 2004,131 (3), 435–44
CrossRef - Ofomaja, A. E.; “Sorptive Removal of Methylene Blue from Aqueous Solution Using Palm Kernel Fibre: Effect of Fibre Dose”; Biochemical Engineering Journal. 2008,40 (1), 8–18.
CrossRef - Rais, A.; and Kumar, R.; “Adsorptive Removal of Congo Red Dye from Aqueous Solution Using Bael Shell Carbon”; Applied Surface Science. 2010, 257 (5), 1628-33.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









