A New Lignan Derivative, Lasiocarpone, from the Stembark of Chisocheton lasiocarpus (Meliaceae)
A. T. Hidayat1,2, Nurlelasari1, F. F. Abdullah1, D. Harneti1, R. Maharani1,2, K. Haikal2, U. Supratman1,2 and M. N. Azmi3
and M. N. Azmi3
1Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Padjadjaran, Jatinangor 45363, Sumedang, Indonesia.
2Central Laboratory, Universitas Padjadjaran, Jatinangor 45363, Sumedang, Indonesia.
3School of Chemical Sciences, Universiti Sains Malaysia, Minden 11800, Pulau Pinang, Malaysia.
Corresponding Author E-mail: unang.supratman@unpad.ac.id
DOI : http://dx.doi.org/10.13005/ojc/3404032
Article Received on : 19-04-2018
Article Accepted on : 01-06-2018
Article Published : 12 Jul 2018
A new lignan derivative, named lasiocarpone (1), was isolated from the stembark of Chisocheton lasiocarpus. The chemical structure of 1 was determined by extensive NMR and MS spectra analyses as well as by comparing with analogue compound from previous studies. Lasiocarpone showed moderate cytotoxic activity against MCF7 breast cancer cells with an IC50 value of 42.5 mM.
KEYWORDS:Chisocheton Lasiocarpus; Lasiocarpone; lignan; Meliaceae; MCF7 Cancer Cell
Download this article as:| Copy the following to cite this article: Hidayat A. T, Nurlelasari N, Abdullah F. F, Harneti D, Maharani R, Haikal K, Supratman U, Azmi M. N. A New Lignan Derivative, Lasiocarpone, from the Stembark of Chisocheton lasiocarpus (Meliaceae). Orient J Chem 2018;34(4). |
| Copy the following to cite this URL: Hidayat A. T, Nurlelasari N, Abdullah F. F, Harneti D, Maharani R, Haikal K, Supratman U, Azmi M. N. A New Lignan Derivative, Lasiocarpone, from the Stembark of Chisocheton lasiocarpus (Meliaceae). Orient J Chem 2018;34(4). Available from: http://www.orientjchem.org/?p=47302 |
Introduction
The Chisocheton plant genera as a second largest plant of Meliaceae family, consisting of 50 plants and widely distributed in the tropics. Previous phytochemical studies of this genera have produced some compounds with interesting biological activity, such as anti-plasmodial limonoid,2 cytotoxic limonoid,3 and anti-inflammatory limonoids,4 NO production inhibitory activity limonoids,5 cell growth inhibitory activity limonoids,6 anti-inflammatory protolimonoids,7 cytotoxic triterpenoid8 and cytotoxic tetranortriterpenoid.9
Our previous phytochemical studies on Chisocheton plants, we had found new limonoids, pentandrice and dysobinol from C. pentandrus10 and C. mocrophyllus11 as well as a triterpenoid-type lanostane from C. cumingianus.12 In the further study, we focus the stembark of C. lasiocarpus that showed significant cytotoxic activity on MCF7 breast cancer cells in vitro.
C. lasiocarpus is up to 20 m high plant and widely distributed in the tropical regions.13 Traditionally, the stembark of C. lasiocarpus are used for treatment of fever and skin diseases.13-15 There is no phytochemical study on C. lasiocarpus previously. In this paper, isolation, structure determination and cytotoxic activity of a new lignan derivative are described.
Material and Method
Instruments
Optical rotation values were measured with a Perkin-Elmer 341 polarimeter. UV spectrum was measured with Shimadzu-1800 spectrophotometer. IR spectrum was measured on Perkin-Elmer 1760X spectrophotometer. Mass spectra was measured with a Qtof HR-MS XEVotm mass spectrometer instruments. NMR data were recorded on a Bruker Topspin spectrometer and used TMS as an internal standard. Column chromatography on SiO2 (Merck). TLC analysis on SiO2 GF254, stain was observed on UV light and heated on the hotplate after spraying with 10% H2SO4 in ethanol.
Plant Material
C. lasiocarpus stembarks were obtained from Bogor Botanical Garden, Indonesia in July 2015. Plant identifications were made from Bogoriense Herbarium, Indonesia. Specimens (No. Bo-1295453) are saved in the Bogoriense Herbarium.
Cytotoxic Assay
Determination of cytotoxic activity is performed according to the procedure described in the previous paper.10 Harvest suspension of breast cancer cells (MCF-7) by centrifugation. Determine the amount and viability cells (with trypan blue exclusion), and resuspend cells with final 4 × 105 cells / mL supplemented with 10% phosphoric buffer solution (FBS) and 1% Penicillin-Streptomycin. Dispense 50 μL of cell suspension (20,000 cells) into all wells on microplate, then incubated for 24 hours. On different microplate samples were prepared. Samples or standards to be measured, diluted in an EMEM medium containing 10% FBS and 1% Penicillin-Streptomycin, then dilutions. The diluted 50 μL sample was transferred into well on the microplate containing the incubated cell. Then re-incubated for 48 hours. After that, a salt 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) (MTS) reagent was introduced to each microplate of 20 μL and incubated for 2-4 hours until the orange formazan was seen. Furthermore, the colored formazan that has been produced measured its absorbance at a wavelength of 570 nm using a multimode reader. The IC50 value was obtained from percentage live cells compared to control (%), versus the tested concentration of compounds (µM).
Results
Extraction and Purification
Dried ground stembarks of C. lasiocarpus (2.1 kg) were soaked with methanol for three days and filtered. Evaporate of the methanol on vacuum produced a concentrated methanol extract (209.4 g) and subsequently partitioned to n-hexane, EtOAc and n-BuOH. A portion of EtOAc (28.3 g) was vacuum liquid chromatographed on SiO2 with n-hexane-EtOAc-MeOH as a gradient solvent to yield seven fractions (I-VII). Fraction IV (3.20 g) was column chromatographed on SiO2 with n-hexane-EtOAc (10:0-1:1) as a developing solvent to give seven subfractions (IVa-IVg). Subfraction IVf (420 mg) was further separated by column chromatography on SiO2 with choroform: methanol (9:1) as a solvent to give five subfractions (IVf.1-5). Subfraction IVf.3 (62.5 mg) was further purified by preparative TLC on SiO2 GF254 with n-hexane: ethyl acetate (8:2) as a solvent to give 1 (5 mg) as a minor compound.
Lasiocarpone (1). Yellowish gum; [α]D20 + 25.1o (c 0.1, CH3OH); UV λmax 280 (e 2.80); IR (KBr) vmax (cm-1): 3394, 3060, 1731, 1720, 1560, 1464, 1024; 1H- (500 MHz) and 13C NMR (125 MHz) in DMSO-d6, Table 1. HR-TOFMS m/z 355.1015 [M+H] + (calcd for C20H18O6, m/z 354.1025).
Discussion
An aqueous methanol extract was concentrated and partitioned to n-hexane, ethyl acetate, and n-butanol. The ethyl acetate fraction was column chromatographed on SiO2 and separated on preparative TLC on SiO2 GF254 produce a new lignan derivative, lasiocarpone (1).
 |
Scheme 1 Click here to View scheme |
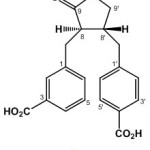
Lasiocarpone (1), [α]25D + 25.1o (c 0.1, CH3OH), was isolated as a yellowish gum with molecular formula, C20H18O6 by high resolution time of flight mass spectra (HR-TOFMS), requiring twelve double bond equivalents. UV absorption of 1 in MeOH exhibited at at 280 nm, indicating the presence on benzene ring. IR spectrum showed peaks at 3394, 1731,1720, 1560 and 1024 cm-1, due to the presence of hydroxyl, carboxyl ester lactone, benzene and ether groups. 1H-NMR spectrum of 1 revealed p-disubstituted benzene ring at δH 7.52 (2H, d, J=6.8 Hz, H-2′, H-6′) and 7.43 (2H, d, J=6.4 Hz, H-3′, H-5′). In 1H-NMR spectrum showed also m-disubstituted benzene ring at δH 7.26 [(1H, d, J=7.1 Hz, H-4), 7.70 (1H, dd, J=7.1, 8.2 Hz, H-5), 7.77 (1H, d, J=8.2 Hz, H-6) and 7.18 (1H, s, H-2)]. In addition, deshielded methylene protons at δH [4.45 (1H, dd, J=7.1, 8.5 Hz), 4.54 (1H, dd, J=8.5, 9.4 Hz)], methine signals at δH [4.67 (1H, dd, J=4.7, 7.1 Hz), 4.19 (1H, m)] and four methylene signals at δH 2.87 (1H, dd, J=5.5, 13.8 Hz), 3.24 (1H, dd, J=7.1, 13.8 Hz), 3.05 (1H, dd, J=10.4, 13.5 Hz) and 3.20 (1H, dd, J=4.7, 13.5 Hz) also were observed and supporting of a dibenzyl butyrolactone-type lignin.16 The 13C-NMR spectrum together with DEPT spectrum of 1 displayed twenty carbon signals, including a lactone (δC 171.9), two carboxyl carbons (δC 167.2, 166.7), four sp2 quartenary carbons, eight sp2 methines, an oxygenated sp3 methylene (δC 65.9), two sp3 methylenes and two sp3 methines, featuring a dibenzyl butyrolactone-type lignan.16,17 A NMR data of 1 are similar those of (2R,3R)-2-(4-hydroxy-3-methoxybenzyl)-3-(3,4,5-trimethoxy)benzylbuyrolactone,17 except the disappear of methoxyl and hydroxyl groups and the appearance of carboxyl group. In order to clarify the position of functional groups, 2D NMR experiments were conducted (Figure 1). Correlations in H2′-H3′, H5′-H6′, H4-H5-H6, H7-H8 and H7′-H8′-H9′, supporting the presence of a 1, 4-disubtituted and a 1,3-disubtituted benzene ring from a dibenzyl butyrolactone-type lignan.
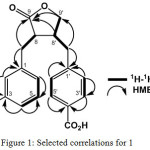 |
Figure 1: Selected correlations for 1 |
All of aromatic protons were correlated to carboxyl and used for assigment of the carboxyl groups were attached in meta and para orientation, respectively. Furthermore, a correlation between an oxygenated methylene at δH 4.45 to C-9 (δC 171.9) and C-8′ (δC 50.3) and a methine proton at δH 4.67 to C-9 (δC 171.9) were used to assign a lactone ring at C-8, C-9, C-9′ and C-8′, which characteristic for butyrolactone-type of lignan. Correlation from methylene protons at δH 3.07 and 2.87 to aromatic carbons at δC 137.8 (C-1), 138.2 (C-1′) and to methine carbons at δC 54.8 (C-8) and 50.3 (C-8′), supporting the dibenzyl butyrolactone-type of lignan. It was clearly confirmed that compound 1 contains a 3-carboxybenzyl at C-9 and 4′-carboxybenzyl unit C-9′ on a butyrolactone skeleton and the relative configuration of the dibenzyl units is trans. A relative stereochemistry of C-8 and C-8′ was supported also by NOESY spectra. There are no crosspeak between H-8 and H-8′ In the NOE spectra, consequently both protons are trans-orientation.
Table 1: NMR data for 1
| Position of C | δC (mult.) | δH (SH., Integral., J=Hz) |
| 1 | 137.8 (s) | – |
| 2 | 129.6 (d) | 7.18 (1H, s) |
| 3 | 134.0 (s) | – |
| 4 | 126.9 (d) | 7.26 (1H, d, 7.1) |
| 5 | 127.6 (d) | 7.70 (1H, dd, 7.1, 8.2) |
| 6 | 128.5 (d) | 7.77 (1H, d, 8.2) |
| 7 | 36.4 (t) | 3.05 (1H, dd, 10.4, 13.5) |
| 3.20 (1H, dd, 4.7, 13.5) | ||
| 8 | 54.5 (d) | 4.67 (1H, dd, 4.7, 7.1) |
| 9 | 171.8 (s) | – |
| 1′ | 138.0 (s) | – |
| 2′ | 131.5 (d) | 7.52 (1H, d, 6.8) |
| 3′ | 128.6 (d) | 7.43 (1H, d, 6.4) |
| 4′ | 134.8 (s) | – |
| 5′ | 128.6 (d) | 7.40 (1H, d, 6.4) |
| 6′ | 131.5 (d) | 7.60 (1H, d, 6.8) |
| 7′ | 36.7 (t) | 2.87 (1H, dd, 5.5,13.8) |
| 3.24 (1H, dd, 7.1, 13.8) | ||
| 8′ | 50.3 (d) | 4.19 (1H, m) |
| 9′ | 65.9 (t) | 4.45 (1H, dd, 7.1, 8.5) |
| 4.54 (1H, dd, 8.5, 9.4) | ||
| 4′-CO2H | 166.7 (s) | – |
| 5-CO2H | 167.2 (s) | – |
This results was supported also by comparing to those of trans-dibenzylbutyrolactone18 and the specific optical rotation of 1 ([α]20 D – 24.1o, c 0.1, CH3OH) is same negative sign to that of the previously reported, (2R,3R)-2-(4-hydroxy-3-methoxybenzyl)-3-(3,4,5-trimethoxy)benzylbuyrolactone ([α]18D -25.1 (c 0.55, EtOH)].17 Therefore, compound 1 was elucidated to be a new dibenzyl butyrolactone-type lignan, (2R,3R)-5-carboxybenzyl-4′-carboxybenzylbutyrolactone and was named lasiocarpone.
Lasiocarpone (1) was checked its cytotoxic activity on MCF7 breast cancer cells according to a method described10,19 and cisplatin (IC50 27.0 µM) was used as a positive control.20 Lasiocarpone displayed an IC50 value of 42.5 mM against MCF7 breast cancer cell, indicating showed moderate cytotoxic activity.
Conclusions
A new dibenzyl butyrolactone-type lignan derivative, lasiocarpone (1), was isolated from the stembark of Chisocheton lasiocarpus as a minor compound. This investigation confirm that Chisocheton genus is capable to produce a lignan derivative.
Acknowledgments
We express sincerely thank to Universitas Padjadjaran for financial support (Academic Leadership Grant 2016-2018 by US). We are thank also to Dr. Yuni Elsa at Central Laboratory, Universitas Padjadjaran, Bandung, Indonesia for MCF7 assay.
References
- Yang, M.H.; Wang, J.S.; Luo, J.G.; Wang, X.B., Kong, L.Y. J. Nat. Prod. 2009, 72, 2014-2018.
CrossRef - Mohamad, K.; Hirasawa, Y.; Litaudon, M.; Awang, K.; Hadi, A.HA.; Takeya, K.; Ekasari, W.; Widyawaruyanti, A.; Zaini, N.C.; Morita, H. Bioorganic and Medicinal Chemistry, 2009, 17, 727-730.
CrossRef - Wong, C.P.; Shimada, M.; Nugroho, A.E.; Hirasawa, Y.; Kaneda, T.; Hadi, A.H.A.; Osamu, S.; Morita, H. J. Nat. Med. 2012, 66, 566–570.
CrossRef - Chong, S,L.; Awang, K.; Martin, M,T.; Mokhtar, M.R.; Chan, G.; Litaudon, M.; Gueritte, F.; Mohamad, K. Tetrahedron Letters, 2012, 53, 5355-5359.
CrossRef - Najmuldeen, I,A.; Hadi, A.HA.; Awang, K.; Mohamad, K.; Ketuly, K.A.; Mukhtar, M.R.; Chong, S.L.; Chan, G.; Nafiah, M.A.; Weng, N.S.; Shirota, O.; Hosoya, T.; Nugroho, A.E.; Morita, H. J. Nat. Prod. 2011, 74, 1313–1317.
CrossRef - Wong, C.P.; Shimada, M.; Nagakura, Y.; Nugroho, A.E.; Hirasawa, Y.; Kaneda, T.; Awang, K.; Hadi, A.H.A.; Mohamad, K.; Shiro, M.; Morita, H. Chem. Pharm. Bull. 2011, 59(3), 407-411.
CrossRef - Yang, M.H.; Wang, J.S.; Luo, J.G.; Wang, X.B.; Kong, L.Y. Bioorganic and Medicinal Chemistry, 2011, 19, 1409-1417.
CrossRef - Yang, M.H.; Wang, J.S.; Luo, J.G.; Wang, X.B.; Kong, L.Y. Can. J. Chem. 2012, 90, 199-204.
CrossRef - Inada, A.; Sukemawa, M.; Murata, H.; Nakanishi, T.; Tokuda, H.; Nishino, H.; Iwashima, A.; Darnaedi, D.; Murata, J. Chem. Pharm. Bull. 1993, 41, 617–619.
CrossRef - Supriatno.; Nurlelasari., Herlina, T.; Harneti, D.; Maharani, R.; Hidayat, A,T.; Mayanti, T.; Supratman, U.; Azmi, M.N.; Shiono, Y. Natural Products Research. 2018, 32, 1-7.
- Nurlelasari.; Katja, D.G.; Harneti, D.; Wardayo, M.M.; Supratman, U.; Awang, K. Chemistry of Natural Compounds. 2017, 53, 83-87.
- Katja, D,G.; Farabi, K.; Nurlelasari.; Harneti, D.; Mayanti, T.; Supratman, U.; Awang, K.; Hayashi, H. Journal of Asian Natural Products Research, 2016, 6, 1-5.
- Bordoloi, M.; Saikia, B.; Mathur, R. K.; Goswami, B. N. Phytochemistry. 1993, 34, 583-584.
CrossRef - Connolly, J.D.; Labbe, C.; Rycroft, D.S.; Taylor, D.A.H. J. Chem. Soc. Perkin Trans. I., 1979, 12, 2959-2964.
CrossRef - Heyne, K., “The Useful Indonesian Plants”, Research and Development Agency, Ministry of Forestry, Jakarta, Indonesia. 1982, 989-1012.
- Kader, A.M.S.; Wisse, J.; Evans, R.; Werff, H.V.D.; Kingston, D.G.I. J. Nat. Prod. 1997, 60, 1294-1297.
CrossRef - Nishibe, S.; Okabe, K.; Hisada. Chem. Pharm. Bull. 1981, 29(7), 2078-2082.
CrossRef - Chenevert, R and Rose, Y.S. Tetrahedron: Asymmetry, 1998, 9, 2827-2831.
CrossRef - Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, R.M. J. Natl. Cancer Inst. 1990, 82, 1107–1112.
CrossRef - Hadisaputri, Y.E.; Pharm, D.; Miyazaki, T.; Suzuki, S.; Yokobori, T.; Kobayashi, T.; Tanaka, N.; Inose, T.; Sohda, M and Kuwano, H. Ann. Surg. Oncol. 2012, 19, S589-S596.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









