Synthesis and Anticancer Effect of 3,4,5-N-Alkyl-Benzamides on Colon Carcinoma HCT- 116 Cells
Jilly Octaria Tagore Chan1, Ade Arsianti2,3,Maurin Marcelia1, Stevano Julio Wijoyo1, Fadilah Fadilah2,3,Rista Putrianingsih2, Norma Nur Azizah3, Hiroki Tanimoto4 and Kiyomi Kakiuchi4
1Medical Student, Faculty of Medicine University of Indonesia.
2Department of MedicalChemistry, Faculty of Medicine, University of Indonesia.
3Drug Development Research Cluster, Indonesia Medical Education and Research Institute (IMERI), Faculty of Medicine, University of Indonesia, Jalan Salemba Raya 6 Jakarta 10430, Indonesia.
4Materials Science, Nara Institute of Science and Technology (NAIST), 8916-5 Takayama-cho, Ikoma, Nara, Japan.
Corresponding Author E-mail: arsi_ade2002@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/340323
Article Received on : April 02, 2018
Article Accepted on : May 04, 2018
The natural phenolic gallic acid has demonstrated a significant inhibition of cell proliferation in a series of cancer cell lines, as well as induced apoptosis in HCT-116 colon cancer cells. This research aims to synthesize six compounds of 3,4,5-trihydroxy-N-alkyl-benzamide derivatives of gallic acid, and investigate its anticancer effect on colon carcinoma HCT-116 cells.Six compounds of 3,4,5-trihydroxy-N-alkyl-benzamide, namely 3,4,5-trihydroxy-N-methyl-benzamide (2); 3,4,5-trihydroxy-N-ethyl-benzamide (3); 3,4,5-trihydroxy-N-butyl-benzamide (4); 3,4,5-trihydroxy-N-sec-butyl-benzamide (5); 3,4,5-trihydroxy-N-tert-butyl-benzamide (6) and 3,4,5-trihydroxy-N-hexyl-benzamide (7), have been successfully synthesized by amidation of carboxyl group of gallic acid with six corresponding alkylamines, respectively. Furthermore, anticancer effect of these six synthesized derivatives on colon HCT-116 cells were examined by MTT assay. Data were analyzed by linear regression method to generate IC50 value. The results will be compared with gallic acid as an original compound and doxorubicine as a positive control.Amidation of gallic acid with six corresponding alkylamines gave desired -N-methyl-, -N-ethyl-, -N-butyl-, -N-sec-butyl-, -N-ters-butyl-, and –N-hexyl benzamide with yield ranging from 18% to 84%. Compared to gallic acid (IC50: 0.05 µM) and doxorubicine (IC50: 0.001 µM), all these six synthesized derivatives showed a lower anticancer effect on colon HCT-116 cells. The strongest anticancer and inhibitory effect on HCT-116 cells has shown by 3,4,5-trihydroxy-N-hexyl benzamide (7) with IC50 value of 0.07 µM. Our results suggested that 3,4,5-trihydroxy-N-hexyl benzamide (7) is a potential to be developed as a promising anti-colon cancer agent.
KEYWORDS:Synthesis; Anticancer; Gallic Acid; 3,4,5-trihydroxy-N-alkyl-benzamide; HCT-116 Cells
Download this article as:| Copy the following to cite this article: Chan J. O. T, Arsianti A, Marcelia M, Wijoyo S. J, Fadilah F, Putrianingsih R, Azizah N. N, Tanimoto H, Kakiuchi K. Synthesis and Anticancer Effect of 3,4,5-N-Alkyl-Benzamides on Colon Carcinoma HCT- 116 Cells. Orient J Chem 2018;34(3). |
| Copy the following to cite this URL: Chan J. O. T, Arsianti A, Marcelia M, Wijoyo S. J, Fadilah F, Putrianingsih R, Azizah N. N, Tanimoto H, Kakiuchi K. Synthesis and Anticancer Effect of 3,4,5-N-Alkyl-Benzamides on Colon Carcinoma HCT- 116 Cells. Orient J Chem 2018;34(3). Available from: http://www.orientjchem.org/?p=45596 |
Introduction
When benign adenomas and polyps transform into malignancies, colorectal cancer is brought about. It is the 3rd most frequently diagnosed cancer and the 4th most common cause of cancer death worldwide.1 According to GLOBOCAN 2012 from the International Agency for Research on Cancer (IARC), the incidence of colorectal cancer in people of both genders was 1,360,602 (9.7 %) while the mortality in that year sum up to as high as 693,933 (8.5%). In Indonesia itself, there was a reported 27,772 (9.3%) colorectal incidence and 18,398 (9.5%) mortality cases in the year of 2012; making this type of cancer reign the 4th against other types of cancer in the aspects of both incidence and mortality in that year.2To determine the appropriate treatment for colorectal cancer, stage of cancer is crucial even though other aspects such as type of tumor, patient’s age, and patient’s degree of health are also taken into consideration. American Joint Committee on Cancer (AJCC) classifies colorectal cancer into 4 distinctive stages along with a 5th “recurrent” stage.3 Surgery is mostly implied for colorectal cancer in its early stages; in estimation, 95% of stage 1 and 65-80% of stage II colorectal cancers can be cured by surgery. Stage III of colorectal cancer is usually treated firstly by tumor resection with administration of adjuvant chemotherapy such as 5-fluorouracil (5-FU) and leucovorin (LV) such as FOLFOX for 6 months afterwards (Longo et al. 2012). Sometimes, oxaliplatin will also be prescribed in addition to minimize the likelihood of recurrence.4,5 Stage IV of colorectal cancer is usually being managed by the combination of therapies which may include surgery, chemotherapy and radiation. Chemotherapy, albeit being a traditional treatment for cancers, possesses undesirable side effects.Chemotherapy drugs namely 5-FU, LV and oxaliplatin often cause neutropenia, diarrhea, vomiting and peripheral neuropathy.6Therefore, new treatments are constantly being developed and researched on so that patients can receive the best treatment possible for their condition. Gallic acid (3,4,5- trihydroxybenzoic acid) is a polyphenolic acid that can be found naturally in plants such as tea leaves, grapes, mango and gall nuts.7 Gallic acid has been shown to be a potent inhibitor of fungi, bacteria and some viruses. Furthermore, according to Morley et al., gallic acid displays chemopreventive activity, which is attributed to its strong apoptosis- inducing and antioxidant effects.8Gallic acid is believed to be apoptosis- inducer through the generation of reactive oxygen species (ROS), Ca2+ influx as well as calmodulin activation.9 Gallic acid’s cytotoxic effects on a few cancer cell lines including colon cancer have been established by multiple studies.10,11In 2016, Abd- Rabou and co-workers tested 5 different concentrations of gallic acid which were 100µM, 50µM, 25µM, 12.5µM, 6.25µM on HCT- 116 cells and all of the results showed significantly decreased (P < 0.01) cell viability; the values being 5.93%, 6.29%, 7.63%, 8.19%, and 22.87% respectively.12 Additionally, gallic acid has been shown to induce apoptosis in cancer cells selectively without doing the same to healthy, normal cells. This terrific finding on the anticancer properties of gallic acid further fuels the interests of researchers to find ways in which the activity could be further enhanced. Owing to the knowledge that gallic acid is a hydrophobic substance, cell membrane permeability is restricted. A study has concluded that an increase in potential cytotoxicity and lipophilicity can be done by modifying the structure of gallic acid by the addition of lipophilic groups.13In the present study, we have done the synthesis of gallic acid derivatives as anti-hepatitis C virus agents.14 Recently, we also done in silico study of gallic acid derivatives as inhibitor of malarial dihydrofolate reductase15, as an inhibitors of hepatitis C virus receptor NS5B16, as an inhibitor of Bcl-xl anti-apoptotic protein of breast cancer17, and as an inhibitor of BRAF colon cancer18.
In this work, as to continue research in developing gallic acid derivatives as an anticancer agent, we focused on the synthesize of 3,4,5-trihydroxy-N-alkyl-benzamides2-7 (Figure 1), andevaluate its anticancer effect on colon HCT-116 cells by MTT cell proliferation assay.
Experimental
Design Structure of Gallic Acid Derivative
Six gallic acid derivatives, compound 2-7 (Figure 1) were designed by replacing carboxyl group of
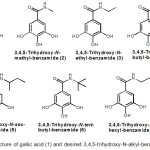 |
Figure 1: Structure of gallic acid (1) and desired 3,4,5-trihydroxy-N-alkyl-benzamides 2-7 |
Gallic acid with a series of corresponding alkylamines to generate 3,4,5-trihydroxy-N-alkyl-benzamides 2-7. Four compounds, namely derivative2, 3, 4 and 7, were designed to have a linear aliphatic carbon chain in N-alkyl benzamide group, whereas compound 5 and 6 were designed to have a branched alphatic carbon chain.
General Experimental Method
All chemical reactions were performed in an oven-dried glassware. Chloroform and methanol in technical grade were purchased from Brataco Indonesia chemical distributor, and were distilled prior to use. Methylamine, ethylamine, butylamine, sec-butylamine, tert-butyl amine, hexylamine and DMF were obtained from Wako Pure Chemical Industries. Gallic acid, doxorubicin, N-(3-dimethylaminopropyl)-n’-ethylcarbodiimide hydrochloride (WSCD.HCl), 1-Hydroxybenzotriazole (HOBt) and N-methyl morpholine (NMM) were purchased from Sigma-Aldrich Chemical Company. Flash column chromatography was carried out using Merck silica gel 60. Reaction and fractions of chromatography were analyzed by Merck precoated silica gel 60 F254 plates. Compounds were visualized by an ultraviolet lamp (254 nm) and by staining with ninhydrin (in EtOH). 1H NMR and 13C NMR spectra were recorded on JEOL JNM-ECP500 (500 MHz) spectrometert with tetramethylsilane (δ 0) and acetone (δ 2.05) as an internal standard. Mass spectra were measured by JEOL JMS-AX 700 spectrometer. Synthesis and cytotoxicity evaluation of 3,4,5-trihydroxy-N-alkyl-benzamides were carried out in Departement of Medical Chemistry, Faculty of Medicine, University of Indonesia. Structure elucidation of the synthesized 3,4,5-trihydroxy-N-alkyl-benzamides were conducted in Graduate School of Materials Science, Nara Institute of Science and Technology (NAIST), Japan. The tested HCT-116 cancer cells are the culture collection of Anatomical Pathology Department, Faculty of Medicine, University of Indonesia.
General Procedure of Amidation
WSCD.HCl (405.0 mg, 3.52 mmol) and NMM (0.77 mL, 7.04 mmol) was added to a solution of gallic acid (300 mg, 1.76 mmol), alkylamine (3.52 mmol) and HOBt (357 mg, 2.64 mmol) in DMF (5 mL). The mixture was stirred at room temperature for 24 h. The reaction was diluted by water (40 mL), and extracted by EtOAc (3 x 25 mL). EtOAc extract was collected, further washed with brine (20 mL), dried over anhydrous MgSO4, filtered and evaporated to give a crude extract. The extract was flash chromatographed on silica gel (gradient elution CHCl3/CH3OH) to give desired 3,4,5-trihydroxy-N-alkyl-benzamide.
3,4,5-trihydroxy-N-methylbenzamide (2)
Derivative 2 (248.0 mg, 75% yield) was obtained from flash column chromatography on silica gel of the crude extract (gradient elution 20:1 to 4:1 CHCl3/CH3OH) as a brown oil. Rf 0.13 (5:1 CHCl3/CH3OH); 1HNMR (500 MHz, acetone-d6): 6.94 (2H, s); 2.80 (3H, d, J = 4.6 Hz); 13CNMR (125 MHz, acetone-d6): 168.3 (s); 146.3 (s); 136.9 (s); 126.3 (s); 107.3 (d); 26.6 (q); HRMS EI+ calcd for C8H9NO4 [M]+: 183.0532, found: 183.0529.
3,4,5-trihydroxy-N-ethyl benzamide (3)
Derivative 3 (68.5 mg, 20% yield) was obtained from flash column chromatography on silica gel of the crude extract (gradient elution 25:1 to 7:1 CHCl3/CH3OH) as a brown oil. Rf 0.31 (5:1 CHCl3/CH3OH); 1HNMR (500 MHz, acetone-d6): 7.03 (2H, s); 3.41-3.34 (2H, m); 1.15 (3H, t, J = 7.4 Hz); 13CNMR (125 MHz, acetone-d6): 167.8 (s); 146.1 (s); 136.8 (s); 126.5 (s); 107.6 (d); 35.2 (t); 15.1 (q); HRMS EI+ calcd for C9H11NO4 [M]+: 197.0688, found: 197.0683.
3,4,5-trihydroxy-N-butyl benzamide (4)
Derivative 4 (237.0 mg, 60% yield) was obtained from flash column chromatography on silica gel of the crude extract (gradient elution 25:1 to 10:1 CHCl3/CH3OH) as a dark brown solid. Rf 0.38 (5:1 CHCl3/CH3OH); 1HNMR (500 MHz, acetone-d6): 7.00 (2H, s); 3.37-3.31 (2H, m); 1.59-1.50 (2H, m); 1.41-1.31 (2H, m); 0.90 (3H, t, J = 7.8 Hz); 13CNMR (125 MHz, acetone-d6): 167.6 (s); 146.0 (s); 136.6 (s); 126.8 (s); 107.5 (d); 40.0 (t); 32.5 (t); 20.7 (t); 14.1 (q); HRMS EI+ calcd for C11H15NO4 [M]+: 225.1001, found: 225.0991.
3,4,5-trihydroxy-N-sec-butyl-benzamide (5)
Derivative 5 (331.4 mg, 84% yield) was obtained from flash column chromatography on silica gel of the crude extract (gradient elution 25:1 to 9:1 CHCl3/CH3OH) as a brown oil. Rf 0.44 (5:1 CHCl3/CH3OH); 1HNMR (500 MHz, acetone-d6): 6.98 (2H, s); 4.05-3.94 (1H, m); 1.63-1.47 (2H, m); 1.16 (3H, d, J = 6.9 Hz); 0.90 (3H, t, J = 8.0 Hz); 13CNMR (125 MHz, acetone-d6): 167.1 (s); 145.9 (s); 136.6 (s); 127.0 (s); 107.5 (d); 67.1 (d); 47.6 (t); 20.6 (q); 11.0 (q); HRMS EI+ calcd for C11H15NO4 [M]+: 225.1001, found: 225.0993.
3,4,5-trihydroxy-N–t-butyl-benzamide (6)
Derivative 6 (69.2 mg, 18% yield) was obtained from flash column chromatography on silica gel of the crude extract (gradient elution 25:1 to 8:1 CHCl3/CH3OH) as a dark brown oil. Rf 0.52 (5:1 CHCl3/CH3OH); 1HNMR (500 MHz, acetone-d6): 6.97 (2H, s); 1.28 (9H, s); 13CNMR (125 MHz, acetone-d6): 167.1 (s); 145.9 (s); 136.6 (s); 127.0 (s); 107.5 (d); 42.6 (s); 29.8 (q); HRMS EI+ calcd for C11H15NO4 [M]+: 225.1001, found: 225.0994.
3,4,5-trihydroxy-N-hexyl-benzamide (7)
Derivative 7 (357.0 mg, 80% yield) was obtained from flash column chromatography on silica gel of the crude extract (gradient elution 25:1 to 5:1 CHCl3/CH3OH) as a yellow solid. Rf 0.33 (5:1 CHCl3/CH3OH); 1HNMR (500 MHz, acetone-d6): 6.99(2H, s); 3.59 (2H, t, J = 5.2 Hz); 1.59-1.52 (2H, m); 1.37-1.23 (4H, m); 0.85 (3H, t, J = 8.2 Hz); 13CNMR (125 MHz, acetone-d6): 167.6 (s); 146.0 (s); 136.7 (s); 126.7 (s); 107.5 (d); 66.9 (t); 56.0 (t); 32.3 (t); 27.3 (t); 23.2 (t); 14.2 (q); HRMS EI+ calcd for C11H15NO4 [M]+: 253.1314, found: 253.1306.
Cytotoxicity Evaluation
Suspension of cells (100 µL) (5000 cells.well) of HCT-116 was dispensed to a 96-well plate. The plate was pre-incubated for 24 h in a humidified incubator containing 5% CO2 at 37oC. The various concentration of tested samples (0-200 mM) were added into the culture media in the plate. The plate was incubated for an appropriate lenght of time (72 h) in the incubator. 10 µL of MTT solution was then added to each well of the plate which was subsequently incubated for 1-4 h in the incubator. This mixture was then measured for absorbance by Elisa reader at 630 nm. This procedure was triplicated for each plate. IC50 value was determined using probit method by plotting the concentration of the sample against percentage of living cells.
Result and Discussion
Synthesis of gallic acid derivatives (3,4,5-trihydroxy-N-alkyl benzamide)
Synthetic pathway of 3,4,5-trihydroxy-N-alkyl-benzamide 2-7 is summarized in Figure 2. As shown, amidation of gallic acid (1) with methylamine, ethylamine, butylamine, sec-butylamine, tert-butyl amine, and hexylamine using a combination of WSCD.HCl/HOBt in the presence of basic NMMafforded the corresponding 3,4,5-trihydroxy-N-alkyl-benzamides, which are 3,4,5-trihydroxy-N– methyl-benzamide (2), 3,4,5-tri-hydroxy-N-ethyl-benzamide (3), 3,4,5-tri-hydroxy-N-butyl-benzamide (4), 3,4,5-tri-hydroxy-N-sec-butyl-benzamide (5),3,4,5-trihydroxy-N-tert-butyl-benzamide (6) and 3,4,5-trihydroxy-N-hexyl-benzamide (7)with a yield ranging from 18% to 84%.
Anticancer effect of 3,4,5-trihydroxy-N-alkyl-benzamides
Anticancer activity of 3,4,5-trihydroxy-N-alkyl-benzamides against colon HCT- 116 cellsis displayed in Table 1. Half maximal inhibitory concentration (IC50) value is utilized to express anticancer activity of the derivatives.The lower IC50 value the higher anticancer activity. As shown in Table 1, compared to gallic acid (IC50: 0.05 µM) and doxorubicine (IC50: 0.001 µM), all these six synthesized derivatives showed a lower anticancer effect on HCT 116 cells.
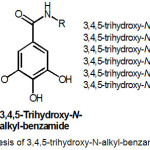 |
Figure 2: Synthesis of 3,4,5-trihydroxy-N-alkyl-benzamides 2-7
|
Except for 3,4,5-trihydroxy-N-butyl-benzamide (4), there is a visible trend of decreasing IC50 value and/or increasing anticancer activity from 3,4,5-trihydroxy-N-methyl-benzamide2(IC50: 2.43 µM), 3,4,5-trihydroxy-N-ethyl-benzamide3(IC50: 1.64 µM), and 3,4,5-trihydroxy-N-hexyl-benzamide7(IC50: 0.07 µM). This signifies that in general, 3,4,5-trihydroxy-N-alkyl-benzamide with longer hydro-phobic carbon chain of –N-alkyl-benzamide group, possess a higher anticancer activity.
However, the fact that all six compounds of 3,4,5-trihydroxy-N-alkyl-benzamides have higher IC50 value than original gallic acid, might suggest that although carboxyl group is classified as being hydrophilic, they are crucial for anti- colorectal cancer activity. Derivative 3,4,5-trihydroxy-N-ethyl-benzamide (3), shows improved anticancer effect on HCT-116 cells when it compared to 3,4,5-trihydroxy-N-methyl-benzamide (2), which implies that an addition of methylene (-CH2) group in 3,4,5-trihydroxy-N-ethyl-benzamide (3), increases its hydrophobicity. Compared to 3,4,5-trihydroxy-N-butyl-benzamide4 (IC50: 3.56 µM) that has linear aliphatic carbon chain in -N-alkyl-benzamide group,3,4,5-trihydroxy-N–sec-butyl-benzamide5 (IC50: 1.34 µM) and 3,4,5-trihydroxy-N–tert-butyl-benzamide 6 (IC50: 0.16 µM)which have a branched aliphatic carbon chain, showed higher anticancer activity against HCT-116 cellss. Moreover, Among all the six synthesized 3,4,5-trihydroxy–N-alkyl-benzamides, derivative 3,4,5-trihydroxy–N-hexyl-benzamide (7) with IC50 of 0.07 µM, shows the greatest potential to be developed as an anti- colorectal cancer agent. Additionally, further studies examining the anti- colorectal cancer properties of 3,4,5-trihydroxy–N-heptyl-benzamide and 3,4,5-trihydroxy–N-octyl-benzamide that possess a longer aliphatic carbon chain than 3,4,5-trihydroxy–N-hexyl-benzamide (7) needs to be conducted.
Table 1. Anticancer activity (IC50 in µM) of 3,4,5-trihydroxy-N-alkyl-benzamides 2-7, gallic acid (1), and doxorubicine against colon HCT-116 cells
|
Compound |
Cytotoxicity (IC50, µM) |
| Gallic acid (1) |
0.05 |
| 3,4,5-trihydroxy-N-methyl-benzamide (2) |
2.43 |
| 3,4,5-trihydroxy-N-ethyl-benzamide (3) |
1.64 |
| 3,4,5-trihydroxy-N-butyl-benzamide (4) |
3.56 |
| 3,4,5-trihydroxy-N-sec-butyl-benzamide (5) |
1.34 |
| 3,4,5-trihydroxy-N-tert-butyl-benzamide (6) |
0.16 |
| 3,4,5-trihydroxy-N-hexyl-benzamide (7) |
0.07 |
| Doxorubicine |
0.01 |
Conclusion
Six compounds of 3,4,5-trihydroxy-N-alkyl-benzamides have been synthesized through amidation of gallic acid with the corresponding alkylamines. Among six synthesized derivatives, 3,4,5-trihydroxy-N-hexyl-benzamide (7) showed the greatest anticancer effect on HCT-116 cells.
Acknowledgements
We wish to express our gratitute to Directorate ofResearch and Public Service (DRPM) and Faculty of Medicine, University of Indonesia for the PITTA (Publikasi Internasional Terindeks untuk Tugas Akhir Mahasiswa) research grant Fiscal year 2017, and to the Graduate School of Materials Science, Nara Institute of Science and Technology (NAIST), Japan, for International Research Collaboration Program (NAIST Global Initiative Program).
References
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Int. J. Cancer. 2010, 127, 2893-2917.
CrossRef - WHO. GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012[Internet]. 2012[updated 2014 Feb 14; cited 2017 Aug 15]. Available from:http://globocan.iarc.fr/Pages/fact_sheets_population.aspx
- Mishra, J.; Dromund, J.; Quazi, S.H.; Karanki, S.S.; Shaw, J.J.; Chen, B. Crit. Rev. Oncol. Hematol. 2013, 86, 232–250.
CrossRef - Longo, D.L.; Kasper, D.L.; Jameson, J.L.; Fauci, A.S.; Hauser, S.L.; Loscalzo, J. Harrison’s Principles of Internal Medicine. 18thed. New York: McGraw-Hill. 2012, 537-538.
- Gramont, A.D.; Figer, A.; Seymour, M.; Homerin, M.; Hmissi, A.; Cassidy, J. J. Clin. Oncol. 2000,18, 2938-2947.
CrossRef - André, T.; Boni, C.; Mounedji-Boudiaf, L.; Navarro, M.; Tabernero, J.; Hickish, T. N Engl. J. Med. 2004, 350, 2343-2351.
CrossRef - Yoon, C.H.; Chung, S.J.; Lee, S.W.; Park, Y.B.; Lee, S.K.; Park, M.C. Joint Bone Spine. 2013, 80, 274-279.
CrossRef - Silva, I.C.; Polaquini, C.R.; Regasini, L.O.; Ferreira, H.; Pavan, F.R. Food Chem. Toxicol. 2017,105, 300-307.
CrossRef - Filipiak, K.; Hidalgo, M.; Silvan, J.M.; Fabre, B.; Carbajo, R.J.; Pineda-Lucena, A. Food Funct. 2014, 5, 381-389.
CrossRef - Esteves, M.; Siquet, C.; Gaspar, A.; Rio, V.; Sousa, J.B.; Reis, S. Arch. Pharm. Chem. Life. Sci. 2008, 341, 164–173.
CrossRef - Khaledi, H.; Alhadi, A.A.; Yehye, W.A.; Ali, H.M.; Abdulla, M.A.; Hassandarvish P. Arch. Pharm. Chem. Life Sci. 2011, 344, 703–709.
CrossRef - Abd-Rabou, A.A.; Shalby, A.B.; Ahmed, H.H. Int. J. Pharm. Bio. Sci. 2016, 7, 584-592.
CrossRef - Dorothea, M.; Arsianti, A.; Liwang, F.; Christian, H.; Panjaitan, H.P. Ann. Oncol. 2016, 17 (suppl 7), 523-532.
- Arsianti, A.; Aoki-Utsubo, C.; Fadilah; Bahtiar, A.; Apriyanto, D.R.; Dwira, S.; Pradisty, N.A.; Tanimoto, H.; Kakiuchi, K.; Sudarmono, P.; Hotta, H.; Paramita, R.I.; Erlina, L. Asian J. Pharm. Clin. Res. 2017, 10, 164-167.
CrossRef - Arsianti, A.; Astuty, H.; Fadilah; Bahtiar, A.; Tanimoto, H.; Kakiuchi, K. Asian J. Pharm. Clin. Res. 2017, 10, 330-334.
CrossRef - Arsianti, A.; Fadilah; Bahtiar, A.; Dwira, S.; Apriyanto, D.R.; Paramita, R.I. Int. J. Chemtech Res. 2017, 10, 111-117.
- Paramita, R.I.; Arsianti, A.; Radji, M. Int. J. Chemtech Res. 2017, 10, 348-355.
- Humaedi, A.; Arsianti, A.; Radji M. Int. J. Chemtech Res. 2017, 10(1), 310-315.

This work is licensed under a Creative Commons Attribution 4.0 International License.









