Magnetic Core–Shell Nanoparticles Containing I3- as a Novel Catalyst for the Facial Synthesis of Imidazole Derivatives in Solvent-Free Conditions
Keivan Ghodrati1, Esmail Korani2 and Manzarbano Asnaashari2
Esmail Korani2 and Manzarbano Asnaashari2
1Department of Chemistry, Kermanshah Branch, Islamic Azad University, Kermanshah. Iran.
2Department of Chemistry, North Tehran Branch, Islamic Azad University, Tehran, Iran.
DOI : http://dx.doi.org/10.13005/ojc/340341
Article Received on : May 30, 2017
Article Accepted on : January 06, 2018
Article Published : 16 May 2018
A novel Fe3O4@SiO2@(CH2)3N+Me3I3- magnetite nanoparticle was prepared and utilized in the synthetic conversion of aldehydes to imidazole derivatives in solvent-free conditions. These functionalized magnetic core–shell nanoparticles (MNPs) were characterized by Standard technique such as TEM, SEM, EDX, FT-IR, XRD, and VSM. The work up of reaction was very simple containing only a magnetic decantation. This novel catalyst could be separated easily and recycled several times without any significant decreasing of catalytic activity.
KEYWORDS:Fe3O4@SiO2@(CH2)3N+Me3I3-; Magnetic Separation; Magnetic Core-Shell; Imidazoles; Solvent-Free
Download this article as:| Copy the following to cite this article: Ghodrati K, Korani E, Asnaashari M. Magnetic Core–Shell Nanoparticles Containing I3- as a Novel Catalyst for the Facial Synthesis of Imidazole Derivatives in Solvent-Free Conditions. Orient J Chem 2018;34(3). |
| Copy the following to cite this URL: Ghodrati K, Korani E, Asnaashari M. Magnetic Core–Shell Nanoparticles Containing I3- as a Novel Catalyst for the Facial Synthesis of Imidazole Derivatives in Solvent-Free Conditions. Orient J Chem 2018;34(3). Available from: http://www.orientjchem.org/?p=45925 |
Introduction
In the past quarter of the century, nanoparticle catalysts have attracted chemists attention because of their unique properties both in the reaction and workup. However, according their simple separation, in addition of their nano size advantages, their application have been developed more and more [1]. In the other hand, most catalysts such as nano particles have some drawbacks in usual separation methods, for instance, filtration or centrifugation. These general methods lead to loss of catalysts and products over the reaction and process steps. The magnetic nano particles can omit or improve many of these drawback [2]. According the advantages of using magnetic nano particles, some of them have been introduced in organic chemistry and catalyst fields [3-15].
Imidazoles and their derivatives are a big class in organic chemistry. They have been introduced and utilized for many years because of their unique biological activity [1]. Imidazoles were usually produced by the reaction between a carbonyl group and 1,2-diamine in the presence of a special reagent or forced reaction condition [2]. The using of numbers of reagents and catalysts indicating the importance of these compounds in organic synthesis. However, the majority of these reagents and catalysts suffer from some drawback such as low yields of the products, tedious work up, toxic reagents, high cost, long reaction time and … . Therefore, introducing a new safe and green reagents are desirable.
In continue with our research about nano catalyst [16], we report herein the synthesis of novel magnetically triiodide catalyst and its application to the synthesis of imidazole derivatives. The synthetic steps are summarized in scheme 1.
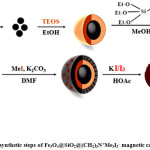 |
Scheme 1: synthetic steps of Fe3O4@SiO2@(CH2)3N+Me3I3– magnetic catalyst. |
The preparation of first magnetite particles as a magnetic core was performed by reaction between ferrous and ferric chloride with NH4OH. An inert layer of SiO2 was coated around previous core. The outer surface modification was made through the grafting of amino propylsilane, conversion of amine group to fourth ammonium group and finally, adding the triiodide anion to nano particle surface. Moreover, this magnetic catalyst was characterized by known appropriate technique such as SEM, TEM, EDX, XRD, FT-IR and VSM. Finally, the produced catalyst was used as a novel catalyst for the synthesis of imidazole derivatives.
Experimental
Preparation of Catalyst
Preparation of Magnetic Nanoparticles (Fe3O4), silica-coated MNPs (Fe3O4@SiO2) and Amino Propyl Modified Silica-Coated Magnetic Nanoparticles (Fe3O4@SiO2@APTES)
These steps were reported before by our researcher team [16e and g].
Preparation of Triiodide Modified Nanoparticle (Fe3O4@SiO2@(CH2)3N+Me3I3–)
The Fe3O4@SiO2@APTES (100 mg) was dispersed in DMF (50 mL) by the ultrasonic treatment for 30 min, MeI (2 mL) and K2CO3 was added to the mixture under vigorous stirring for 6 h. An external magnet was used to separation of Fe3O4@SiO2@(CH2)3N+Me3I– from the solution. Then the produced nano particles washed with water several times and dried in air. Afterward, this nano particle was dispersed in ethanol (20 ml) by ultrasonic treatment and a solution of I2 in ethanol was added to dispersed solution and was stirred for one day. The resulting dark violet nano catalyst was collected by an external magnet and washed with 5 mL ethanol.
General Procedure
A solution of substrate (1 mmol), aryl aldehyde (1 mmol), and Fe3O4@SiO2@(CH2)3N+Me3I3– MNPs (0.007 gr) was heated at 100°C under magnetic stirring for the requested reaction time. The complication of reaction was demonstrate by TLC. The EtOH (10 mL) was added and the reined nano particles was collected by an external magnet. The mixture was poured into ice-water (30 mL) and the pure heterocyclic product was filtered, washed with ice-water, and dried.
Results and Discussion
Characterization of Magnetic Nanoparticle
TEM Analysis
The size and morphology of the prepared novel catalyst were characterized by TEM (Fig. 1). The TEM observation indicates all prepared nano particles have almost spherical core–shell structure (Fig. 1). The size of magnetic core is less than 20 nm and the diameter of all layers a shell are around 30 nm.
 |
Figure 1: TEM images of (a) Fe3O4@SiO2 (b)Fe3O4@SiO2@(CH2)3N +Me3I3–. |
SEM Analysis
The Scanning Microscope Electronic (SE) images indicate size and morphology of prepared nano particles (fig. 2). According this data, all nano particles have a spherical shape with a large surface area. These images show that Fe3O4 is spherically with nano dimensions less than 15 nm, but when TEOS coated The Fe3O4 the average sizes increase to 20 nm (fig. 2b). Furthermore, the SEM observation of Fe3O4@SiO2@APTES determined a recognizable layers on Fe3O4 magnetic core (fig. 2c). Finally, fig. 2d shows the structure of Fe3O4@SiO2@(CH2)3N+Me3I3– as core shell structure with average size of particles less than 50 nm.
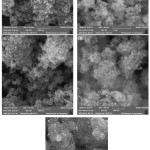 |
Figure 2: SEM images of prepared nano particles: (a) Fe3O4 (b) Fe3O4@ SiO2 (c) Fe3O4@SiO2@ NH2 (d) Fe3O4@ SiO2@ N(CH3)2I– (d) Fe3O4@ SiO2@ N(CH3)2I3– |
X-Ray Diffraction
XRD pattern of pure magnetic nano catalyst are showed in fig 3. The most intense peak shows the average sizes of nano particles according Debye–Scherrer’s formula D = 0.9λ/β cosθ, (D= the average crystalline size; λ= X-ray wavelength (1.542 Å); β= the angular line width of half-maximum intensity; θ= Bragg’s angle in degree) [19, 20]. Based on this data averages sizes of synthesized nano particles are less than 50 nm that confirm the SEM and TEM data.
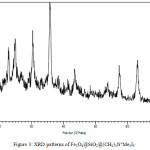 |
Figure 3: XRD patterns of Fe3O4@SiO2@(CH2)3N+Me3I3– |
EDS Analysis
Duo to EDS patterns, clearly show the presence of N, C, O, Si, Fe and I (fig.4). The presence of Fe indicate iron oxide in nano articles. The higher intensity signals of Si and O, in addition, shows the grafting of SiO2 layer to magnetic core. The signal of C proves the presence of this element in the structure of nano particle. Likewise, the N and I signal demonstrate the alkylated ammonium group and triiodide anion respectively.
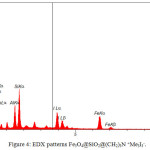 |
Figure 4: EDX patterns Fe3O4@SiO2@(CH2)3N +Me3I3–. |
FTIR Spectrosocpy
In the fig. 5a-cthe FT-IR spectra of all prepared nano particles are showed. The Fe-O bond showed a significant signal in 585 cm-1 [21]. It is noticeable that Si-OH and Si-O-Si groups were confirmed by bands at 804, 951 and 1086 cm−1. The stretching vibration of -OH group was confirmed by a broad band at 3300 – 3500 cm−1 (fig. 5b). The significant bands at 2965 and 2850 cm−1 belong to the stretching vibration of C-H bond of propyl amine group of APTES, which prove the successful grafting of this on silica layer. Furthermore, a band at 1440 cm−1 belong to the tertiary amine group. Overall, these results confirm that this novel nano particles successfully prepared.
 |
Figure 5: FT-IR spectra: (a) Fe3O4 @ SiO2 MNPs; (b) APTES coated Fe3O4 MNPs and (c) Fe3O4@SiO2@(CH2)3N+Me3I3–. |
Magnetic Properties
The magnetization curve of Fe3O4 , silica coated Fe3O4 and Fe3O4@SiO2@(CH2)3N+Me3I3– are presented in fig. 6a-c. This kind of characterization would be called vibrating sample magnetometer (VSM). As can be seen from the diagrams, the magnetization of bare Fe3O4 MNPs is about 60.0 emu/g at room temperature [23]. When the layers were added, the saturation magnetization would be decrease more and more. In fact, the saturation magnetization of Fe3O4@SiO2@(CH2)3N+Me3I3– would be around 40 emu/g, which is lower than the bare one. Decrease of the saturation magnetization result from the creation of a silica shell around the Fe3O4 core.
 |
Figure 6: VSM of prepared nano particles at room temperature of (a) Fe3O4 MNPs; (b) Fe3O4@SiO2 MNPs; and (c) Fe3O4@SiO2@(CH2)3N+Me3I3–. |
Optimization of Nano Catalyst for Imidazole Synthesis
The reaction process is simple and fast and proceeded in excellent yields of products. At the first step, the conversion reaction carried out in different protic and aprotic solvents and the product yields compared to solvent free condition (Table 1).
Table 1: Effect of different solvent on product yield for the synthesis of 2-(4-chlorophenyl)-1H– benzimidazole
|
Entry |
Solvent a |
Time(min)/Yield(%) |
|
1 |
CH3CH2OH |
60/ trace |
|
2 |
CH3CH2OCH2CH3 |
60/ trace |
|
3 |
CH2Cl2 |
60/ trace |
|
4 |
THF |
60/ trace |
|
5 |
Toluene |
60/ trace |
|
6 |
CH3CN |
60/ trace |
|
7 |
DMF |
60/14 |
|
8 |
None b |
60/36 |
|
9 |
None c |
60/72 |
|
10 |
None d |
15/94 |
The reactions were carried out with 4-chlorobenzaldehyde (1mmol), orth-phenylene diamine (1mmol), and nano catalyst (0.07 g). a) reflux condition, b) in solvent- free condition at 50˚C, c) in solvent- free condition at 75˚C, d) in solvent- free condition at 100˚C
The imidazoles preparation reaction was performed in the both solvent and solvent free conditions. As regard the first, a verity of different solvents were utilized for this conversion, such as diethyl ether, ethanol, dichloromethane, THF, toluene, acetonitrile, and DMF. Although, very low yield of products were obtained in the presence of DMF as solvent, but no one performed a noticeable yields. As can be seen from the table 1 only solvent free condition is the best case for this reaction. Likewise, the catalyst amount’s effect was explored on reaction time and yields table 2). Although, in the absence of this catalyst no products were found, but there are two basic trends in the presence of different amount of catalyst. The yield of products went up by increasing the catalyst amount from 0.025 to 0.07 g. But with higher amount of catalyst the yield of products were leveled off.
In general, all data show significantly that the best reaction condition is the solvent free at 100°C in the presence of 0.07g of periodate magnetic nano catalyst.
Table 2: Catalyst amounts effect on the preparation of 2-(4-chlorophenyl)-1H– benzimidazole
|
Entry |
Catalyst(g) |
Time(min)/ Yield(%) |
|
1 |
0.025 |
15/29 |
|
2 |
0.05 |
15/85 |
|
3 |
0.07 |
15/94 |
|
4 |
0.10 |
15/94 |
|
5 |
– |
15/ – |
Reaction of 4-chloro benzaldehyde (1mmol), 1,2- diamino benzene (1mmol), and catalyst in solvent- free conditions at 100˚C.
The results of condition’s optimization were used in the reaction of a variety of aldehyde derivatives with 1,2-diamino benzene to synthesis of some heterocyclic compounds (table 3).
Synthesis of Imidazole, Thiazole and Pirimidine Derivatives using Fe3O4@SiO2@(CH2)3N+Me3I3–
As can be seen from the table 3, there are 15 examples for catalytic conversion of aldehyde to the corresponding heterocyclic compounds using this novel catalyst containing I3–. The yields are excellent and the procedure is very simple. Likewise, the work up is easy too. The catalyst is readily separated by only using an external magnet.
![]()
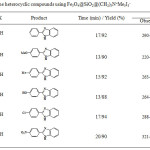 |
Table 3: Synthesis of some heterocyclic compounds using Fe3O4@SiO2@(CH2)3N+Me3I3– |
Catalyst Recovery and its Stability
The reaction of 4-chlorobenzaldehyde and o-phenylene diamine in the presence of the catalytic amount of Fe3O4@SiO2@(CH2)3N+Me3I3– was performed for demonstrate recoverability of this catalyst. The catalyst was recycle and reused for five times without any losing of its activity. In fact, this unique nano catalyst could be recycled and reused times and times (fig. 7). On the other hands, the catalyst shows very good shelf life too. This novel catalyst last for months. So, it can be stored for months without significant decline in its activity.
 |
Figure 7: Reusability of catalyst in |
Conclusions
In this manuscript, we herein report a novel magnetic nano catalyst containing periodide (Fe3O4@SiO2@(CH2)3N+Me3I3–) as a green, efficient and recoverable nano catalyst. The separation of this catalyst from reaction mixture is very simple too. It could be isolated using an external magnet vessel. The imidazole, thiazole, and pirimidine derivatives could be easily prepared using this novel nano catalyst in excellent yields.
Acknowledgments
Financial support from the Islamic Azad University of Kermanshah and north Tehran are gratefully acknowledged.
References
- (a) W.A. Denny, G.W. Rewcastle, B.C. Baugley, Journal of MedicineChemistry33 (1990) 814;
(b) D.A. Horton, G.T. Bourne, M.L. Smythe, Chemical Reviews103 (2003) 893;
CrossRef
(c) R. Cedillo-Rivera, O. Munoz, Journal of Medical Microbiology37 (1992) 221;
CrossRef
(d) G. Navarrete-Vazquez, R. Cedillo, A. Hernandez-Campos, L. Yepez, F. Hernandez-Luis, J. Valdez, R. Morales, R. Cortes, M. Hernandez, R. Castillo, Bioorganic & Medicinal Chemistry Letters11 (2001) 187;
CrossRef
(e) X. Bu, L.W. Deady, G. J. Finlay, B. C. Baguley, W.A. Denny, Journal of MedicineChemistry44 (2001) 2004. - (a) A. Loupy, A. Petit, J. Hamelin, Synthesis 9(1998) 1213;
CrossRef
(b) I. Bhatnagar, M.V. George, Tetrahedron 24 (1968) 1293;
CrossRef
(c) K. Bahrami, M.M. Khodaei, F. Naali, Journal of Organic Chemistry 73 (2008) 6835;
CrossRef
(d) Menteea, E.; Yılmaza, F.; Mutlua, F.; Kahveci, B., Journal of Chemical Research, (2015), 39(11), 617-676;
CrossRef
(e) M.A. Weidner-Wells, K.A. Ohemeng, V. N. Nguyen, S.Fraga-Spano, M.J. Macielag, H.M. Werblood, B.D.Foleno, G.C. Webb, J.F. Barrett, D. Hlasta, Journal Bioorganic Medicine Chemistry Letters 11(2001) 1545;
CRossRef
(f) H. Fujioka, K. Murai, Y. Ohab, A. Hiramatsu, Y. Kita, Tetrahedron Letters46 (2005)2197;
CrossRef
(g) M. Zendehdel, A. Mobinikhaledi, H. Alikhani, N. Jafari, Journal of the Chinese Chemical Society 57(2010) 683.
CrossRef - C.O. Dalaigh, S.A. Corr, Y.G. Ko, S.J. Connon, Angewandte Chemie International Edition 46 (2007) 4329.
CrossRef - (a) A. Schatz, O. Reiser, W.J Stark, Chemistry-A European Journal16 (2010) 8950;
CrossRef
(b) A.H. Lu, E.L.Salabas, F. Schuth, Angewandte Chemie International Edition 46 (2007) 1222.
CrossRef
(c) P. Wang, A.G. Kong, W.J. Wang, H.Y. Zhu, Y.K. Shan, Catalysis Letters 135 (2010) 159–164.
CrossRef
(d) S. Zavar, Arabian Journal of Chemistry, 10 (2017) S67–S70.
CrossRef - C. Duanmu, L. Wu, J. Gu, X. Xu, L. Feng, X. Gu, Catalysis Communications, 48 (2014) 45–49.
CrossRef - J. Zhang, Y. Wang, H. Ji, Y. Wei, N. Wu, B. Zuo, Q. Wang, Journal of Catalysis 229 (2005) 114.
CrossRef - (a) B. Panella, A. Vargas, A. Baiker, Journal of Catalysis 261 (2009) 88.
CrossRef
(b) H. Emtiazi, M.A. Amrollahi, B.F. Mirjalili, Arabian Journal of Chemistry, 8 (2015) 793–797.
CrossRef - J Safaei-Ghomi, S. H. Nazemzadeh, H. Shahbazi-Alavi, Catalysis Communications, 86 (2016) 14–18.
CrossRef - (a) R. Baharfar, S. Mohajer, Catalysis Letters 146 (2016) 1729–1742.
CrossRef
(b) R.K. Sharma, Y. Monga, A. Puri, Catalysis Communications 35 (2013) 110–114.
CrossRef
(c) A. Teimouri, A.N. Chermahini, Arabian Journal of Chemistry, 9 (2016) S433–S439.
CrossRef - (a) B. Baruwati, D. Guin, S.V. Manorama, Organic Letters 9 (2007) 5377.
CrossRef
(b) B. Dam, M. Saha, A.K. Pal Catalysis Letters 145 (2015) 1808–1816.
CrossRef
(c) Ch.X. Liu, Q. Liu, C.Ch. Guo Catalysis Letters 138 (2010) 96–103.
CrossRef - F. Zhang, J. Jin, X. Zhong, S. Li, J. Niu, R. Li, J. Ma, Green Chemistry 13 (2011) 1238.
CrossRef - (a) S. Ceylan, C. Friese, C. Lammel, K. Mazac, A. Kirschning, Angewandte Chemie International Edition 47 (2008) 1.
CrossRef
(b) S. Alabbad, S.F. Adil, M.E. Assal, M. Khan, A. Alwarthan, M.R.H. Siddiqui, Arabian Journal of Chemistry, 7 (2014) 1192–1198.
CrossRef - A. Maleki, Z. Alrezvani, S. Maleki, Catalysis Communications, 69 (2015) 29–33.
CrossRef - K. Fujita, S. Umeki, M. Yamazaki, T. Ainoya, T. Tsuchimoto, H. Yasuda, Tetrahedron Letters 52 (2011) 3137.
CrossRef - (a) M. Bhardwaj, B. Jamwal, S. Paul Catalysis Letters 146 (2016) 629–644.
CrossRef
(b) S. Luo, X. Zheng, J.P. Cheng, Chemical Communications 44 (2008) 5719.
CrossRef
(c) J. Safari, S.H. Banitaba, S.D. Khalili, Arabian Journal of Chemistry, 5 (2012) 419–424.
CrossRef - (a) N. Haghnazari, C. Karami, K. Ghodrati, F. Maleki, Int. Nano Lett., 1 (2011) 30-33.
(b) K. Ghodrati, S. H. Hosseini, R. Mosaedi, C. Karami, F. Maleki, A. Farrokhi, Z. Hamidi, Int. Nano Lett., 3 ( 2013)1-4.
CrossRef
(c) C. Karami, H. Ahmadian, M. Nouri, F. Jamshidi, H. Mohammadi, K. Ghodrati, A. farrokhi, Z. Hamidi, Catalysis Communications 27 (2012) 92–96.
CrossRef
(d) K. Ghodrati, A. Farrokhi, C. Karami, Z. Hamidi, Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry, 45 (2015) 15–20.
CrossRef
(e) A. Farrokhi, K. Ghodrati, I. Yavari, Catalysis Communications 63 (2015) 41–46.
CrossRef
(f) C. Karamia, H. Mohammadi, K. Ghodrati, H. Ahmadian, F. Jamshidi, M. Nouri, N. Haghnazarie, Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry 45 (2015) 271–276.
CrossRef
(g) E. Dezfoolinezhad, K. Ghodrati, R. Badri, New J. Chem., 40 (2016) 4575.
CrossRef - X. Liu, Z. Ma, J. Xing, H. Liu, Journal of Magnetism and Magnetic Materials 270 (2004) 1.
CrossRef - W. Stober, A. Fink, E. J. Bohn,Journal of Colloid and Interface Science 26 (1968) 62.
CrossRef - T.Z. Yang, C.M. Shen, H.J.Gao, The Journal of Physical Chemistry B109 (2005) 23233.
CrossRef - J. Giri, S.G.Thakurta, J.Bellare, A.K. Nigam, D. Bahadur, Journal of Magnetism and Magnetic Materials 293 (2005) 62.
CrossRef - R.D. Waldron, Physical Review 99 (1955) 1727.
CrossRef - F.H. Chen, Q. Gao, J.Z. Ni, Nanotechnology 19 (2008) 165103.
CrossRef - V.S. Zaitsev, D.S. Filimonov, I.A. Presnyakov, R.J. Gambino, B. Chu, Journal of Colloid and Interface Science 212 (1999) 49.
CrossRef - P. Oxley, W.F. Short, Journal of Chemical Society (1947) 497.
CrossRef - B. George, E.P. Papadopoulos, E. P. Journal of Organic Chemistry 42 (1977) 441.
CrossRef - P. Gogoi, D. Konwar, Tetrahedron Letters 47 (2006) 79.
CrossRef - K. Bougrin, A. Loupy, M. Souuflaoui, Tetrahedron 54 (1998) 8055.
CrossRef - R. Kumar, Y.C. Joshi, Eur. J. Chem. 4 (2007) 606.
- L.S. Gadekar, B.R. Arbad, M.K. Lande, Chinese Chemical Letters 21 (2010) 1053.
CrossRef - T. Yoshiyuki, Y. Kazuaki, H. D. Koagakubu and K. Hokoku, Chem. Abstr., 1980, 93, 45-49204537k.

This work is licensed under a Creative Commons Attribution 4.0 International License.









