Antimicrobial Activity of Silver Nanoparticles Synthesis by Green Method from Artemisia Monosperma
1Department of Chemistry, Faculty of Science, Northern Border University, Saudi Arabia.
2Department of Ecophysiology, Desert Research Center, Mathaf El-Mataria, 15753 Egypt.
DOI : http://dx.doi.org/10.13005/ojc/340331
Article Received on : March 28, 2018
Article Accepted on : April 19, 2018
Article Published : 10 May 2018
Nowadays there is world concentration with synthesis of nanoparticles especially silver and gold nanoparticles, by green method for use in many application, exporting antimicrobial effect, Artemisia monosperma is medicinal plant have potent, anticancer, antispasmdial activity, The study aim to synthesis AgNPs from aqueous plant extract by green method to avoid the chemical toxicity biosynthesis and assay the antibacterial activity of synthesis AgNPs. The nanoparticles produced were further characterized using, UV, IR, and Transmission Electron Microscopy (TEM). TEM analysis confirm the synthesis of silver nanoparticles, (AgNPs) which have spherical shape and an average size of 17 nm. Antibacterial assay of capped AgNPs against Gram-positive and Gram-negative bacteria, revealed that AgNPs had good antibacterial activity than aqueous extract under condition prepared, each naonparticle and aqueous extract haven't activity toward Candida albicans. GC-MS of plant extract investigate many important compounds, flavonoid (Kumatakenin), alkaloid (Aspidospermidin-17-ol) and other organic compounds (methyl-jasmonate and verbenol). These results showed that AgNPs can efficacy used in biotechnological and biomedical applications.
KEYWORDS:Gram-Positive; Gram-Negative Bacteria; Silver Nanoparticles; Artemisia Monosperma
Download this article as:| Copy the following to cite this article: Elsharkawy E. R. Antimicrobial Activity of Silver Nanoparticles Synthesis by Green Method from Artemisia Monosperma. Orient J Chem 2018;34(3). |
| Copy the following to cite this URL: Elsharkawy E. R. Antimicrobial Activity of Silver Nanoparticles Synthesis by Green Method from Artemisia Monosperma. Orient J Chem 2018;34(3). Available from: http://www.orientjchem.org/?p=45708 |
Introduction
From very dangerous Gram negative bacteria Pesudomonas aeruginosa cause infection due to longer stay in hospital and it is resistance to many antimicrobial because it have ability to survive under drastic condition, Pesudomonas aeruginosa is most suitable known agent used for study control for nosocomial hospital infection [1,2].
Among great health challenge antimicrobial of green synthesis of nanoparticles from natural source, [3] the high ratio of volume to surface and its chemical and physical properties of nanoparticles have important role of excellent antimicrobial activity, [4] silver nanoparticles are characteristic with good antibacterial, antifungal and antiviral [5,6].
The synthesis of silver nanoparticles by green method is preferred due to less expensive and and could suitable for large scale of synthesis nanoparticles. Many plant extract can use for synthesis nanoparticale as posses many antioxidant which act as reducing agent Plant extracts possess many antioxidants, which act as reducing agents [7].
Genus Artemisia family asteraceae, comprises about 300 species distributed over all world the genus represented by five species in northern region Saudi Arabia (A. monosperma, A. scoparia, A. judica and A. sibri). A. monosperma is green shrub let 50-70-cm high, flowering from spring season and extended to September [8].
Secondary metabolite of Artemisia species and essential oil are widely medicinally used for many year. Studied was aimed to use the medicinal plant Artemisia monosperma for green synthesis of nanoparticle and study the antimicrobial activity of synthesis nanoparticles against gram positive and gram- negative bacteria.
Material and Methods
Plant Material
Fresh plant Artemisia monosperma were collected in spring 2017 year from Tabok, North region in Saudi Arabia, and identified in Biology department, faculty of science, Northern Border University, the sample were kept in Herbarium of faculty of science girl section.
Preparation of Plant Extract
10 gram of powdered plant sample (Aerial part) of Artemisia monosperma was boiling with 100 ml deionized water for 15 min, then filtered and complete the filtrate to 100 ml deionized water and kept at 0°C for synthesis nanoparticles .
Synthesis of silver nanoparticles AgNPs
Synthesis of the AgNPs, take 0.2, 0. 4, 0.6, 0.8, 0.5, 1.0 and 1.5 ml of plant extract and added to the 5 ml, (10-3 M) AgNO3 to each of them the volume was adjusted to 10 ml by de-ionized water. Final concentration of Ag+ was 1×10-4 M, the Ag+ ion was reduced to Ag0 and color of the solution changed from yellow, brownish-yellow to dark brown, within 2 hours at room temp 37°C and neutral pH condition.
Characterization of Silver Nanoparticles Synthesis
The characterization and confirmation of AgNPs synthesis was conducted at Advanced Materials and Nano scale Research Laboratory, Ain shams university by UV-Vis Spectrophotometer UV–vis spectral analysis was conducted using a Perkin Elmer-Lambda 950– UV-visible spectrometer. UV–vis spectra of AgNPs and Ag ions were recorded in range from 300 to 500 nm. [ 9].
TEM Analysis of AgNPs
Transmission Electron microscopy (TEM) was used to examine the size and morphology of synthesis nanoparticles, the image was done on a JEOL-1200 JEM for the TEM measurements.
Infrared Spectrometry Spectra
Infrared spectroscopy were measured on a perkin-Elemer 1000 fourier transforms inferared spectrometer, silver nanoparticles was centrifuge at 16.330 recf for 20 minutes and dried, then mixed with KBr powder for measurement.
Analysis of plant Extract by GC-MS
The constituents of the aqueous extract analyzed by GC-MS as described by Elsharkawy and shiboop 2018, [10]. Compounds identified by comparison of their retention indices (RI), (C9 to C24 n-alkane mixture) and mass spectra with those reported in the literature [10].
Antimicrobial Activity Assay
Antimicrobial activity assay of the synthesized AgNps, nanoparticles was studied against the growth of Gram Positive bacteria (Bacillus subtitles ATCC 6633, Staphylococcus aureus ATCC 25923), Gram negative bacteria (E. coli ATCC 25922, and Pseudomonas aeruginosa ATCC 27858) and Fungi (Candida albicans ATCC 10231) in comparison with that of aqueous Artemisia monosperma extract by using the standard agar well diffusion technique. as method described by Abdallah et al., 2016 [11].
Results and Discussion
The plant Artemisia monosperma was used, to synthesis nanoparticle under facial condition gust by addition of aqueous plant extract to 5 ml of aqueous solution AgNO3, at different concentration from 0.2 ml to 1.5 ml of aqueous extract, the solution color change gradually from yellow, brownish-yellow to dark brown gust after 2 hours at a normal condition of temperature 37°C and neutral ph, as shown in fig 2, indicated the preliminary formation of nanoparticle. The formation of AgNPs was monitored by UV-Viable spectrophotometer, where silver nanoparticles characteristic by absorption under viable range 300-500 [9].
 |
Figure 1: Picture of plant Artemisia monosperma |
UV-Visible
To confirm the formation and stability of synthesis nanoparticle were assayed by UV-Viable spectrophotometer, figure 3 shows the UV-Viable spectra of AgNPs formed at constant concentration of AgNO3 (10-3M). The spectra shows the change of color of AgNPs with changing the plant extract concentration. As is clear from the inset in figure 3 a the color changed from pale yellow to dark brown depending on the extract concentration. As shown in figure 2, UV –vis spectra showed that in the range of low amounts of the leaf extract (0.2–1.5 ml in 10 ml solution), the absorption spectra exhibit a gradual increase of the absorbance accompanied with a shift in the λ max of SPR band absorption peak from 450 to 478 nm. Further increasing the concentration of plant extract with constant amount of AgNO3, the λmax was shifted to longer wavelengths. The inset UV-vis spectrum was related to the Ag ions before the bio-reduction event, in which an absorption band appeared at 290 nm.The appearance of the above SPR peak at 460 nm, along with absence of 290 nm absorption band indicate of the successful synthesis of AgNPs under experimental conditions. Such a characteristic SPR peak for AgNPs has been reported to predominantly appear in the range of 300-500 nm [12].
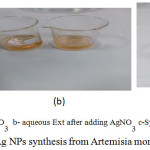 |
Figure 2: Color change of Ag NPs synthesis from Artemisia monosperma |
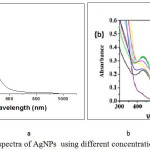 |
Figure 3: UV –Vis spectra of AgNPs using different concentration of Plant Extract. |
FT IR Spectrum
FTIR spectrum was used to identify the possible function groups of biomolecules in the plant extract that might be responsible for bioreduction and coating of AgNPs,. The spectrum of the FTIR spectra of plant extract showed a broad band at 3408 cm-1. This band attributed to the OH groups in the biomolecules. The IR bands at 2920 and 2852 cm-1 due to C–H stretching vibration modes in hydrocarbon chains. The IR bands at 1181 and 1725 cm-1were characterized as C–O and C=O stretching of the carbonyl functional group. The stronger band at 1642 cm-1was characterized as C=O of the amide groups of protein, 1516cm−1 due to stretching vibrations of –C–C– in aromatic rings. In the case of Ag NPs, a large shift in the absorbance peak with decreased band intensity was observed from 3408.4 to 3404.7 cm−1 and 1382.2 to 1320 cm−1 . The spectra also illustrate a prominent shift in the wave numbers corresponding to amide (1652.5–1600 cm−1), indicate the presence of free amino group which may be interacted with AgNPs surface maintain the stability of nanoparticles. the IR spectrum, at fig 4, indicating the capping of the nanoparticles with the extract constituents.
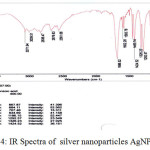 |
Figure 4: IR Spectra of silver nanoparticles AgNPs |
TEM Analysis of AgNPs
The formation of nanopaerticles was confirmed by TEM (Transsimision electron microscopy), the image of TEM of silver NPs depicted in figure 3, indicate the shape, morphology and size of synthesis AgNPs, the size was in the range of 13- 18 nm, and the shape was spherical and irregular.
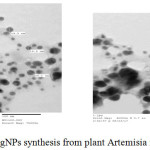 |
Figure 5: TEM image of AgNPs synthesis from plant Artemisia monosperma |
Antimicrobial Activity
The results of the antibacterial evaluation are presented in table 1. In general, silver Nanopartocles synthesis from Artemisia monosperma aqueous extract showed higher antibacterial against both of gram positive and gram negative than plant aqueous extract.
The mean zone of inhibition of Artemisia monosperma ranged between 2.5.0±0.0 to 3.5±0.5mm, for Gram-positives, and 1.5±0.5 to 2.0±1.0 for Gram-negatives for aqueous extract which is week as compared with nanoparticles which shows the mean inhibition zone ranged between 7.0±0.5 to 8.0 ±0.0 for gram negative, among all tested bacteria the most susceptible bacteria was pseudomonas aeruginosa. Elshsharkawy et al., [14]. reported that Mean zone of inhibition of the essential oils of Artemisia monosperma ranged between 20.5±0.0 to 23.0±0.5 mm, for Gram-positives, and 16.5±0.5 to 10.0±1.0 for Gram-negatives. The most susceptible bacteria was Satphylococcus aureus. The antimicrobial activity of silver is well known. In its nanometric form, this characteristic is accentuated. Due to their size, AgNPs can enter cells and inhibit enzymatic systems in the respiratory chain of some bacteria and thereby alter their DNA synthesis
The solubility of NPs in aqueous medium was enhance antibacterial activity where the a plant aqueous extract show moderate antibacterial activity . The antimicrobial activity of the prepared nanoparticles were studied and the activity was found to depend on the concentration of nanoparticles and their sizes. Other studies confirm our result and recommended by use AgNPs as alternative control of microorganism with less risk of toxicity to mammalian cells [15].
Table 1: Antimicrobial activity of the tested plant’s against some microorganism
| Tested compound | Mean zone of inhibition (mm) | ||||
| G- bacteria | G+ bacteria | Fungi | |||
| bacillus subtilis | Staphylococcus aureus | Pesudomonasaeruginosa | Echerichia coli | Candida albicans | |
| Ag NPs | 8.0 ±0.0 | 7.0±0.5 | 8.5±1.5 | 8.0±0.5 | 0 |
| Aqueous plant extract | 1.5±0.5 | 2.0±1.0 | 3.5±0.5 | 2.5±0.5 | 0 |
| Erythromycin (15 µg/disc) | 6.0±0.0 | 18.0±1.0 | 7.5±0.5 | 6.0±0.0 | 2.2±0.0 |
Phytochemical Analysis
Preliminary phytochemical analysis of aqueous extract showed the plant contain more active constituents, flavonoid, terpene, steroid ,coumarin and alkaloid compound, so further analysis of extract was studied to investigate and identified on the chemical compounds in the plant which responsible on antimicrobial activity, aqueous extract was fractionated between methanol and water, methanol fraction was analysis by GC-MS and illustrated the presence of 13 compounds shown in table 2, while water fraction show the presence of protein and glucoside. [16].
Table 2: compounds identified by GC-MS from methanol fraction
| compounds |
RI |
RRI |
% |
| Dasycarpidan-methanol, acetate |
650 |
671 |
0.05 |
| Verbenol |
812 |
813 |
7.54 |
| Azafrin |
501 |
514 |
0.05 |
| Olean1-2-ene-3,15,16,21,22,28-hexol |
439 |
472 |
0.02 |
| Ursodeoxycholic acid |
439 |
472 |
0.55 |
| Nerolidyl acetate |
807 |
819 |
0.50 |
| Xanthoxylin |
854 |
860 |
3.95 |
| Methyl jasmonate |
857 |
871 |
0.64 |
| Aspidospermidin-17-ol |
652 |
675 |
0.02 |
| psi.,.psi.Carotene, |
433 |
436 |
0.04 |
| Pterin -6-carboxylic acid |
669 |
695 |
0.07 |
| 5,4′-dihydroxy-3,7-dimethoxyflavone (Kumatakenin) |
651 |
666 |
2.7 |
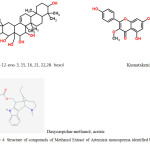 |
Figure 6: Structure of compounds of Methanol Extract of Artemisia monosperma identified by GC-MS |
Conclusion
This work presented synthesis consisting of a reduction of the silver ions using a Artemisia monosperma extract for the first time. The silver, nanoparticles synthesis were confirmed using different techniques. In present study, antimicrobial of synthesized AgNPs by green methods from aqueous extract of plant A. monosperma, as well as plant extract alone were assayed against gram positive, gram negative bacteria and Candida albicans, the results indicate the higher activity against gram positive and negative bacteria of synthesis AgNPs than aqueous plant extract, where the solution acting as capping ligand. GC-MS of plant extract shown many important flavonoid (Kumatakenin), alkaloid (Aspidospermidin-17-ol) and other organic compounds(methyl-jasmonate and verbenol). The results support the importance of further study to confirm our result to use these AgNPs as antibiotic and investigate the combination of silver nanoarticles with antibiotic against resistance.
Acknowledgments
The author gratefully acknowledgment the approval and support of this research by the grant no: SCI-2017-1-7-F-7157 from the deanship of Scientific Research of Northern Border University, Arar, Saudi Arabia
References
- Bergogne-Bérézin E. 2004. Pseudomonas and miscellaneous Gram-negative bacilli. In: Cohen J, Powderly WG, editors. Infections Disease. 2nd ed. Philadelphia, PA: Mosby;. pp. 2203–2217.
- Pollack M. Pseudomonas aeruginosa. In: Mandell GL, Douglas RG Jr, Bernett JE, editors. Principles and Practice of Infectious Diseases. 5th ed. Philadelphia: Churchill Livingstone; 2000. pp. 2310–2317.
- Riley MA, Robinson SM, Roy CM, Dennis M, Liu V, Dorit RL. 2012. Resistance is futile: the bacteriocin model for addressing the antibiotic resistance challenge. Biochem Soc Trans.;40(6):1438–1442. [PubMed]
CrossRef - Singh R, Nalwa HS. 2011. Medical applications of nanoparticles in biological imaging, cell labeling, antimicrobial agents, and anticancer nanodrugs. J Biomed Nanotechnol.;7(4):489–503. [PubMed]
CrossRef - Rai MK, Deshmukh SD, Ingle AP, Gade AK. 2012. Silver nanoparticles: the powerful nanoweapon against multidrug-resistant bacteria. J Appl Microbiol. 112(5):841–852.[PubMed]
- Morones JR, Elechiguerra JL, Camacho A, 2005. The bactericidal effect of silver nanoparticles. Nanotechnology. ;16(10):2346–2353. [PubMed]
- Mittal A. K. , Y. Chisti, U.C. Banerjee. 2013. Synthesis of metallic nanoparticles using plant extracts. Biotech Adv. 31 346–356.
CrossRef - Guetat A, Al-Ghamdi FA, Osman AK 2016. The genus Artemisia L. in the northern region of Saudi Arabia: essential oil variability and antibacterial activities. Nat Prod Res. 2017 31(5):598-603.
CrossRef - Masoud E., A.Al-Hajry M and Al-Marrani. A. 2016 Antibacterial Activity of Silver Nanoparticles Synthesized by Sidr (Ziziphus spina- Christi) Leaf Extract against Pathogenic Bacteria, Int.J.Curr.Microbiol.App.Sci (2016) 5(4): 226-236 226.
- Elsharkawy R. E. and Shiboob M. 2017. Antioxidant activity of phenolic and alkaloid fractions accumulated in Artemisia judica and Artemisia herba alba, Journal of Natural Remedies ,17 (4) |. www. informaticsjournals.com/index.php/jnr.
- Abdallah E.M., Elsharkawy E.R. and Ed-dra A. 2016. Biological activities of methanolic leaf extract of Ziziphus mauritiana. Bioscience Biotechnology Research Communications 9(4): 605-614.
- Khali M.M.H. l, EH Ismail, KZ El-Baghdady, Mohamed, D. 2014. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab J Chem. 71131–1139.
- kaur, G. ; Verma R. ,Rai D., Rai, Rai, S. 2012, Plamamon-enhanced luminescence of Sm complex using silver nanoparticles in polyvinyl alcohol, J.Lumin,132:1683-1687.
- Ghareeb M. A. Shoeb, H. M. Madkour,L. A. Refahy, M. A. Mohamed and. Saad, A. M. 2014. Antioxidant and Cytotoxic Activities of Flavonoidal Compounds from Gmelina arborea Roxb Global Journal of Pharmacology 8 (1): 87-97.
- ElsharkawyE.R., Ed-dra A., Alghanem S., AbdallahE. 2018. Comparative studies of chemical composition, antimicrobial and antioxidant of Essential oil of some species from genus Artemisia; (Under publish).
- Mujeeb Khan, Shams Tabrez Khan, Merajuddin Khan, Syed Farooq Adil, Javed Musarrat, Abdulaziz A Al-Khedhairy, Abdulrahman Al-Warthan, Mohammed Rafiq H Siddiqui, Hamad Z Alkhathlan , 2014. Antibacterial properties of silver nanoparticles synthesized using Pulicaria glutinosa plant extract as a green bioreductant, Int J Nanomedicine. ; 9: 3551–3565.

This work is licensed under a Creative Commons Attribution 4.0 International License.










