Synthesis of Silver Nanoparticles in the Presence of Polyethylene Glycol and Their Electrochemical Behavior at A Graphite Electrode by Cyclic Voltammetry
Darya O. Perevezentseva1, Elena A. Vaitulevich1 and Vladimir I. Bimatov2
1Tomsk Polytechnic University, 634050, Russia, Tomsk, Lenin Ave., 30.
2Tomsk State University, 634050, Russia, Tomsk, Lenin Ave., 36.
Corresponding Author E-mail: dop@tpu.ru
DOI : http://dx.doi.org/10.13005/ojc/340267
Article Received on : September 14, 2017
Article Accepted on : January 01, 2018
Was to study influence of silver nanoparticles (AgNPs) obtainment conditions on their electrochemical activity. They are as follows methods of initiation such as heating and irradiation, the stabilizer introduction order and the amount of reducing agent. Methods of spectrophotometry, cyclic voltammetry and transmission microscopy. It has been shown that in the absence of polyethylene glycol (PEG), a mixture of AgNPs of various shapes is formed: polyhedral, spherical, nanorods with an average particle size of up to 40 nm. In the presence of PEG, AgNPs of triangular shape of smaller size up to 20 nm predominate, which is caused by the reduction of silver ions on the surface of the nucleai. In the presence of PEG, the maximum of the absorption band of AgNPs is shifted to the short-wavelength region by 10 nm compared to the absorption bands of AgNPs obtained in the absence of PEG. It has been found that in the presence of PEG the height of the anode maximum of AgNPs obtained by irradiation is 3.7 times higher than that of AgNPs obtained by heating. The potential of this anodic maximum shifts to 100 mV to area of positive potential values, the one of the cathodic maximum shifts to 100 mV to area of negative potential values, which is associated with the formation of more stable AgNPs oxides obtained by irradiation. It has been shown that the order of introduction of the reagents determines parameters of electrochemical signals of the AgNPs. The electrochemical activity of AgNPs does not depend on the order of introduction of the reagents during their preparation. However, the potential of the anode maximum of AgNPs depend on it. It has been established that the amount of reducing agent affects their electrochemical activity. AgNPs obtained with PEG in a molar ratio [Ag]:[C6H5O73-] = 1:5 have the most electrochemical activity compared to AgNPs obtained in the equimolar ratio of the reagents. The anode maximum of these AgNPs splits into two: the shoulder at E = 0.4 V and the maximum at E = 0.6 V. The appearance of the shoulder is associated with the oxidation of smaller AgNPs. Thus, the size and form of AgNPs determins their electrochemical activity. The synthesis conditions control their electrochemical behavior caused by a change in the state of their surface.
KEYWORDS:Silver Nanoparticles; Electrochemical Behavior; Polyethylene Glycol; Sol; Gel Synthesis
Download this article as:| Copy the following to cite this article: Perevezentseva D. O, Vaitulevich E. A, Bimatov V. V. Synthesis of Silver Nanoparticles in the Presence of Polyethylene Glycol and Their Electrochemical Behavior at A Graphite Electrode by Cyclic Voltammetry. Orient J Chem 2018;34(2). |
| Copy the following to cite this URL: Perevezentseva D. O, Vaitulevich E. A, Bimatov V. V. Synthesis of Silver Nanoparticles in the Presence of Polyethylene Glycol and Their Electrochemical Behavior at A Graphite Electrode by Cyclic Voltammetry. Orient J Chem 2018;34(2). Available from: http://www.orientjchem.org/?p=44144 |
Introduction
Currently the metal-based nanomaterials are widely used in electronics, optics and chemistry, as well as in pharmacology, medicine [1], making and packing of food [2]. They are used as initial materials for synthesis, catalytic materials, sensors [3], conductors [4], detergents and antimicrobial coatings [5]. Due to their active usage, there is a growing interest to the works describing the processes of obtaining metal nanoparticles (MNPs) [6] and investigating their properties [7, 8]. This fact can be explained by their high reactivity and properties that are different from those of high-mass materials, caused by the size of the particles less than 100 nm, their chemical composition, surface area and surface structure. Any of these properties can be controlled by the conditions during synthesis of MNPs [9–11]. The synthesis of AgNPs in aqueous medium is an important field of study because of their bactericidal and healing capabilities. The citrate method of obtaining AgNPs is known; its main drawback being varying shape of resulting particles. To increase stability of silver sols while retaining their high chemical and biological activity for a long time stabilizers are used, such as thiols, PEG and others [12–15]. Their presence prevents aggregation of the particles and allows controlling their shape and size. Although there are some overviews on how the synthesis conditions affect properties of silver particles, there is no detailed data showing their electrochemical characteristics.The purpose of this work was to study influence of AgNPs obtainment conditions on their electrochemical activity. They are as follows methods of initiation such as heating and irradiation, the stabilizer introduction order and the amount of reducing agent.
Materials and Method
Reagents
The experiment uses silver nitrate Merc 99.8%, sodium citrate (Alfa–Aesar, Aldrich Chemical Co, 99,98%), sodium hydroxide, Merc ≥ 99.0%, (Mrw=16000, Aldrich Chemical Co). All solutions were made using distilled water (0.15·106 Ohm).
AgNPs Synthesis
AgNPs were obtained using chemical reduction of silver nitrate with sodium citrate in water medium with controlled pH 9 and air atmosphere while stirring continuously by the magnetic mixer. Deaeration was done with nitrogen for 15 minutes. The initial AgNO3 concentration in solutions under study was 1·10-4 М. The molar ratios between silver cations and sodium citrate were 1:1, 1:3 and 1:5, respectively. The initial mixtures were heated until the color changed to brown. The processes were conducted at room temperature, visible light radiation, while heating in the range of 50–100 °С and introducing stabilizing additive of 0.1 % PEG into the silver sol during different stages of the synthesis. PEG was chosen as stabilizer because of its water solubility, lack of toxicity, thermal stability and complexation properties.
Optical Absorption Spectra
The spectrophotometry was used for control the formation of AgNPs and adsorption on their surface. Optical absorption spectrums were measured using Curry 80 and 2800 UV/VIS Spеctrophotometer in an optical cell 1 cm thick at room temperature in the range from 200 to 800 nm.
Microscopy
The shape and size of the particles were determined by using transmission electron microscopy (TEM) in JSM-5500 electron microscope (Japan). The samples were prepared by applying a drop of reactive system on the copper grid covered by amorphous carbon; the samples were air-dried afterwards.
Electrochemical Activity of AgNPs
The electrochemical curves of AgNPs were registered using the cyclic voltammetry with TA-2 analyzer (Russia) connected to a personal computer. Three-electrode cell was used in the process; the indicator electrode was graphite electrode (GE); auxiliary and reference electrodes were chloride silver electrodes. The electrochemical curves were registered in the range of potentials from -1.0 V to +1.5 V, at potential change rate of 100 mV/s in 0.1 M NaOH solution, which was used as a supporting electrolyte.
AgNPs Precipitation on GE
AgNPs were precipitated on the surface of GE from the silver sol during the period tacc=300 s with accumulation potential Еacc = 0.8 V. The electrode was then removed from the solution, washed in the doubly distilled water and placed in the cell filled with the supporting electrolyte solution 0.1 M NaOH for electrochemical measurements.
Results and Discussions
The microphotographs of AgNPs are shown in Fig.1. The analysis of the particles size has shown that it depends on synthesis method within the range of 5–47 nm.
![Figure 1: TEM of AgNPs obtained with the molar ratio [Ag]: [C6H5O73-] = 1:3, without PEG (a), with PEG (b).](http://www.orientjchem.org/wp-content/uploads/2018/04/Vol34No2_Syn_Dar_fig1-150x150.jpg) |
Figure 1: TEM of AgNPs obtained with the molar ratio [Ag]: [C6H5O73-] = 1:3, without PEG (a), with PEG (b). |
According to the TEM, AgNPs are the mixture of isolated nanoparticles of varying shape when PEG is either present (а) or not (b). The sols of Ag without PEG mostly have polyhedral aggregated particles (fig.1a), with PEG most particles are triangular (fig.1b). Without PEG the average size of 35 nm are for polyhedral particles, 30 nm for spherical particles and 47 nm for nanorods. The average size of particles with PEG is 20 nm for triangular particles, 15 nm for particles of hexagonal shape and 20 nm for cubic particles. As can be seen in Fig.1b, adding PEG during the synthesis of AgNPs at lower temperature of 50°С does not change the growth mechanism and allows to obtain particles 10–20 nm in size due to reduction of silver ions on the surface of the nuclei; according to [16].
Fig.2 shows optical (UV-visible) absorption spectra of the silver sols depending on the methods used to obtain AgNPs. All AgNPs have the wide absorption band in 300–500 nm area with the absorption maximum located from 400 to 420 nm depending on the method of AgNPs synthesis (the method of initiation). This band corresponds to AgNPs plasmon resonance 10–40 nm wide. If sodium citrate concentration is increased from 1:1 (curve (···), fig.2a) to 1:5 (curve (-) fig.2a) during reduction of silver there is the increase absorption at band maximum (λ = 420 nm) for AgNPs obtained without PEG. Shifting a position of the absorption maximum in these spectra is not observed.
The increase in Plasmon resonance can be explained by the increase in silver reduction speed and increase of the amount of AgNPs created.
The similar dependence of increasing absorption band intensity from the citrate sodium concentration is observed under simultaneous PEG addition and heating the reaction mixture to 50°С irrespective of the order of adding reactants.
 |
Figure 2: UV–vis absorption spectra of AgNPs obtained without PEG (synthesis temperature 100°С) (a), with PEG the molar ratios AgNO3: Na3C6H5O7 = 1:3 (b). |
When analyzing the optical spectrum of the silver sol synthesized with PEG at room temperature and irradiated with visible light, a absorption band with λmax ≈ 410 nm can be seen (Fig.2), which indicates that smaller particles are being formed.
The position of the maximum of AgNPs absorption band obtained with PEG and heating to 50 °С shifts to more short-waved area (λmax ≈ 402 nm) (Fig.2b, curve (-)), which indicates that smaller particles are being formed.
PEG prevents the physical contact between AgNPs, and, therefore, blocks the particle aggregation. Broadening of spectrum and the appearance of the long-wave wing regardless of the method of initiating (Fig. 2b) are observed. This fact is explained with AgNPs imperfect crystalline structure or decreasing adsorption layer [15].
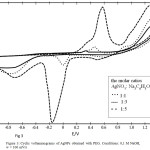 |
Figure 3: Cyclic voltammograms of AgNPs obtained with PEG. Conditions: 0,1 M NaOH, w = 100 mV/s |
![Figure 4: Cyclic voltammograms of AgNPs obtained with reagent molar ratio [Ag]:[C6H5O73-] = 1:3 with PEG under different initiation condition. Conditions: 0.1 M NaOH, w = 100 mV/s](http://www.orientjchem.org/wp-content/uploads/2018/04/Vol34No2_Syn_Dar_fig41-150x150.jpg) |
Figure 4: Cyclic voltammograms of AgNPs obtained with reagent molar ratio [Ag]:[C6H5O73-] = 1:3 with PEG under different initiation condition. Conditions: 0.1 M NaOH, w = 100 mV/s |
In the first series of experiments the electrochemical activity of AgNPs obtained with PEG was studied based on reagents’ molar ratio. As shown in fig. 3 if the amount of reductant used to obtain AgNPs is increased 5 times AgNPs electrochemical activity increases 26. The potential of anodic maximum shifts to 200 mV more area of positive potentials, which indicates the formation of more stable oxides of silver [16] in a reducing medium [17]. There is a maximum at E = 0.3 V on the anodic branch of cyclic curve (-) (fig.3) AgNPs obtained with PEG in a molar ratio [Ag]:[C6H5O73-] = 1:1. There are no maxima on the cathodic branch of the cyclic curve (-) (fig.3) which indicates the complete AgNPs reduction to metallic silver. Similar results were obtained in the absence of PEG [18].
The AgNPs obtained with PEG in a molar ratio [Ag]:[C6H5O73-] = 1:5 have the most electrochemical activity (curve (-), fig. 3). There is the splitting anodic maximum into two on anodic branch of the cyclic curve. They are as follows: a shoulder at E1 = 0.4 V, and a maximum at E2 = 0.6 V. Furthermore, a wave is observed at E = 1.0 V. The appearance of the shoulder is due to the oxidation of AgNPs of different size, which is consistent with the literature data [19]. The appearance of the first maximum is due to oxidation of silver in the silver oxide (I). The appearance of the wave corresponds to oxidation of silver oxide (I) to silver oxide (II), according to [20].
It is found that irradiating AgNPs with light (curve (- -), fig.4) increases height of the anodic maximum by 3.7 times compared with anodic maximum of AgNPs obtained by heating (curve (···), fig.4). The height of the cathodic maximum is increased by 4.2 times. The potential of the anodic maximum shifts to 100 mV to area of positive potential values, the one of the cathodic maximum shifts to 100 mV to area of negative potential values. These data are due to the formation of larger aggregates of silver. An analogical effect is found for gold nanoparticles in [21]. It is found that electrochemical activity of AgNPs does not depend on the order of addition of reagents during their obtainment (curves (-), (···), fig 4). However, the order of reagents’ addition affects the position of the maximа on the potential scale.
If AgNPs were obtained by first introduction of a stabilizer PEG and then a reducing agent an offset of anodic maximum is observed per 100 mV to an area of positive potentials compared with potentials AgNPs obtained by first introduction of a reducing agent and then a stabilizer PEG. A potential of cathodic maximum of these particles simultaneously shifted to 100 mV to the area of negative values compared with potentials AgNPs obtained by first introduction of a reducing agent and then a stabilizer PEG. These data indicate the formation of silver oxide in different oxidation states. They have a greater stability on the surface AgNPs obtained when stabilizer is introduced into the solution before reductant.
Conclusion
It is shown that the producing conditions nanoparticles determines their formation. In the absence of PEG, a mixture AgNPs is obtained: the particles are polyhedral, spherical, nanorods with the average particle size of 40 nm. With PEG the triangular silver nanoparticles predominate; their average size is 20 nm, which is caused by the silver ion reduction on the surface of the nuclei.
It is found that in the optical spectrum the absorption band peak position depends on the composition of the reaction mixture and initiation conditions. There is a maximum at λ = 420 nm in the optical spectrum in the absence of PEG. The addition of PEG to the reaction mixture with simultaneous reduction to room temperature and irradiation with visible light results in a shift of the maximum of the absorption band is 10 nm, which indicates a decrease AgNPs size. The addition of PEG in reaction mixture at temperatures of 50 °С leads to a shift of the maximum to λ = 402 nm.
The electrochemical behaviour of AgNPs stabilized with PEG at the graphite electrode is studied according to the conditions of their obtainment. They are as follows: the amount of reducing agent, the method of initiation of the formation of nanoparticles and the introduction order of reagents in the reaction mixture. It is established that AgNPs obtained in the presence of PEG in a molar ratio [Ag]:[C6H5O73-] = 1:5 have the most electrochemical activity. There is the shoulder on the anodic branch of the cyclic curve of AgNPs which suggests the formation of silver nanoparticles with various sizes onto the graphite electrode in the presence of PEG. The electrochemical activity of AgNPs at the initiation of which was used by light irradiation is greater the electrochemical activity of AgNPs obtained by heating, which is associated with the formation of the most stable oxide of silver (I). AgNPs electrochemical activity does not depend on the order of introduction of reagent during their preparation. It is found that the most stable silver nanoparticles are formed when introducing reductant afterwards.
Acknowledgements
The research is founded Tomsk Polytechnic University enhancing competitiveness program grants.
References
- Wiley, B.; Sun, Y.; Mayers, B.; Xia, Y. Shape-Controlled Synthesis of Metal Nanostructures: The Case of Silver. European J. of Chemistry, 2005, 11, 454–463.
CrossRef - Karam, L.; Jama, C.; Dhulster, P.N. Study of surface interactions between peptides, materials and bacteria for setting up antimicrobial surfaces and active food packaging. J. Mater. Environ. Sci., 2013, 4(5), 798-821.
- Aleixandre, M.; Gerboles, M. Review of Small Commercial Sensors for Indicative Monitoring of Ambient Gas. Chemical engineering transaction, 2012, 30, 169-174.
- Cunningham, J.C.; Kogan, M.R.; Tsai, Y.J.; Luo, L.; Richard, L.; Crooks, R.M. Paper-based Electrochemical Detection of Silver Nanoparticle Labels by Galvanic Exchange. ACS Sens, 2016, 1, 40-47.
CrossRef - Durán, N.; Marcato P.D.; Conti, R.D.; Oswaldo L.A.; Fabio, T.M.; Marcelo, B. J. Potential Use of Silver Nanoparticles on Pathogenic Bacteria, their Toxicity and Possible Mechanisms of Action. Braz. Chem. Soc., 2010, 21, 949-959.
CrossRef - Krutyakov, Yu.A.; Kudrinskiy, A.A.; Olenin, A.Yu. Lisichkin, G.V. Synthesis and properties of silver nanoparticles: advences and prospects. Russian Chem. Rev., 2008, 77(3), 242-269.
CrossRef - Korshunov, A.; Heyrovský, M. Dispersion of silver particles in aqueous solutions visualized by polarography/voltammetry Electrochimica Acta, 2009, 54(26), 6264-6268.
CrossRef - Bilankohi S.M., Ebrahimzadeh M., Ghaffary T. Study of the Properties of Au/Ag Core/Shell Nanoparticles and its Application. Indian Journal of Sci. Technol. 2015, 8, 31-33.
CrossRef - Leontidis, E.; Kleitou, K.; Leodidou, T.K.; Bekiari, V.; Lianos, P. Gold Colloids from Cationic Surfactant Solutions. 1. Mechanisms That Control Particle Morphology. Langmuir, 2002, 18(9), 3659-3668.
CrossRef - Meng, X.K.; Tang, S.C.; Vongehr, S.A. A Review on Diverse Silver Nanostructures. J. Mater Sci. Technol. 2010, 26, 487-522.
CrossRef - Meng, C.; Li-Ying; Jian-Tao Han; Jun-Van Zhang; Zhi-Yuan Li; Dong-lin Qian. Preparation and Study of Polyacryamide-Stabilized Silver Nanopanoparticles through a One-Pot Process. Journal of Fudan Universiry, 2006, 45, 34-38.
- Song, K.C.; Lee, S.M.; Park, T.S.; Lee, B.S. Preparation of colloidal silver nanoparticles by chemical reduction method. Korean J. Chem. Eng., 2009, 26, 153-155.
CrossRef - Liu, J.K.; Yang, X.H.; Tian, X.G. Preparation of Silver/Hydroxyapatite Nanocomposite Spheres. Powder Technology, 2008, 184, 21-24.
CrossRef - Luo, C.C.; Zhang, Y.H.; Zeng, X.W.; Zeng, Y.W.; Wang, Y.G. The role of poly(ethylene glycol) in the formation of silver nanoparticles. J. Colloid. Interface Sci., 2005, 288, 444-448.
CrossRef - Popa, M.; Pradell, T.; Crespo, D.; Calderon-Moreno, J.M. Stable silver colloidal dispersions using short chain polyethylene glycol. Colloids and Surf A: Physicochem. Eng. Aspects, 2007, 303, 184–190.
CrossRef - Bansal, V.; Li, V.; O’Mullane, A.P.; Bhargava, S.K. Shape dependent electrocatalytic behaviour of silver nanoparticles. Cryst. Eng. Comm., 2010, 12, 4280-4286.
CrossRef - Meretukov, M.A. Gold: chemistry, mineralogy, metallurgy, Moscow, 2008.
- Perevezentseva, D.O.; Gorchakov, E.V.; Petrushin, M.S.; Khisamutdinov, I.S.; Bimatov, V.I. In: AIP Conference Proceedings, 2016, Vol. 1772, pp. 0200051-0200055.
- Mohan, S.; Okumu, F.; Oluwafemi, O.; Matoetoe, M.; Arotiba, O. Electrochemical Behaviour of Silver Nanoparticle-MWCNTs Hybrid Nanostructures Synthesized via a Simple Method. Int. J. Electrochem. Sci., 2016, 11, 745–753.
- Perevezentseva, D.O.; Gorchakov, E.V.; Oskina, Yu.A. In: Key Eng. Mat., 2016, Vol. 712, pp. 117-122.
CrossRef - Brainina, K.Z.; Galperin, L.G.; Vikulova, A.V.; Galperin, A.L. The Effect of the System Polydispersity on Voltammograms of Nanoparticles Electrooxidation. J. Solid State Electrochem, 2005, 17(1), 2013, 43-53.

This work is licensed under a Creative Commons Attribution 4.0 International License.









