Synthesis and Antibacterial Activity 1-Monolaurin
Febri Odel Nitbani1 , Jumina2, Dwi Siswanta3, Eti Nurwening Sholikhah4 and Dhina Fitriastuti4
, Jumina2, Dwi Siswanta3, Eti Nurwening Sholikhah4 and Dhina Fitriastuti4
1Department of Chemistry, Faculty of Science and Engineering, Universitas Nusa Cendana, Kupang, Indonesia.
2Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Gadjah Mada, Yogyakarta, Indonesia.
3Department of Pharmacology and Therapy, Faculty of Medicine, Universitas Gadjah Mada Yogyakarta, Indonesia.
4Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Islam Indonesia, Yogyakarta, Indonesia.
Corresponding Author E-mail: febri_nitbani@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/340233
Article Received on : January 04, 2018
Article Accepted on : March 20, 2018
An improvement of method for synthesizing 1-monolaurin from lauric acid and glycerol has been done. The reaction was carried on mol ratio between lauric acid and glycerol 1:1 at 130°C for 6 h with variation of pTSA catalyst of 2.5%, 5%, 7.5% (w/w of lauric acid).The purification of 1-monolaurin was conducted only by extracting with hydroalcoholic solution. The product of 1-monolaurin was obtained as a white solid with 100% of purity from variation of 2.5% and 5% of pTSA catalyst with 43.54% and 27.89% yield, respectively. 1-Monolaurin could inhibit the growth of S. aureus and E. coli bacteria at 500 µg/mL of concentration.
KEYWORDS:Lauric Acid; Glycerol; Monolaurin; Antibacteria
Download this article as:| Copy the following to cite this article: Nitbani F. O, Jumina J, Siswanta D, Sholikhah E. N, Fitriastuti D. Synthesis and Antibacterial Activity 1-Monolaurin. Orient J Chem 2018;34(2). |
| Copy the following to cite this URL: Nitbani F. O, Jumina J, Siswanta D, Sholikhah E. N, Fitriastuti D. Synthesis and Antibacterial Activity 1-Monolaurin. Orient J Chem 2018;34(2). Available from: http://www.orientjchem.org/?p=44710 |
Introduction
Indonesia is the biggest coconut production country beside Malaysia and India but diversification of coconut based product is still low1. Currently, in Indonesia or other countries, coconut oil mostly was developed as drug such as virgin coconut oil (VCO). Thus, the diversification of coconut based product is still necessary.
Coconut oil contained lauric acid with relative percentage of methyl laurate of 52%2. Lauric acid as white solid could be synthesized through base hydrolysis of methyl laurate with 84% of yield. The other product that can be derived from coconut oil is monoacylglycerol of medium chain fatty acid such as 2-monolaurin3, 1-monokaprin4 and
1-monomiristin5. Monolaurin compound is known to have antimicrobial activity (antibacterial, antivirus and antifungi) 6,7,8,9. Monolaurin also has antivirus activity i.e. active to dissolve lipid and phospholipid in the outer layer of virus so that the virus will be degraded 5.
Monolaurin has a promising antimicrobial activity but the compound is not easy to be synthesized and relatively expensive. The attempts to synthesize monolaurin has been done by Pareira et al10and Langone et al11 by esterification reaction of lauric acid and glycerol using Lipozyme IM 20 catalyst but the yield is still low (26.8%). Review article written by Nitbani et al. (2015)12 stated that, the successful application of MCM-41 hybrid materials as the catalysts in the esterification of glycerol with either lauric acid or oleic acid. By maintaining the ratio between methyl groups to the sulphonic acid and applying the optimum conditions of the catalyst, monolaurin and monoolein compounds can be generated with a yield of 63% and 45% respectively.
Synthesis of 1-monolaurin through esterification reaction of lauric acid and glycerol using sulfuric acid catalyst and solvent-free has been done by Widiyarti et al.(2009)7. Purification of 1-monolaurin compound was done by using column chromatography with silica gel as stationary phase and a mixed solvent of n-hexane and ethyl acetate as mobile phase. The reaction was carried out using lauric acid: glycerol (1:1), 5% of catalyst at 130 °C for 6 h and solvent-free. The synthesized 1-monolaurin has 31.05% of yield and 91% of purity.
In this current paper, research of synthesis and purification method of 1-monolaurin was done. The reaction usedpTSA catalyst rather than H2SO4 and purification of the product employed liquid-liquid extraction. This synthesis innovation is expected to increase the yield and purity of the 1-monolaurin compound. The product will also be tested for its antibacterial activity against S. aureus and E. coli bacteria.
Experimental Section
Materials
Chemicals used in this experiment were lauric acid2, glycerol, pTSA, n-hexane, ethyl acetate, ethanol and distilled water. All chemicals, except distilled water which was obtained from Laboratory of Fundamental Chemistry UniversitasGadjahMada, were purchased from E. Merck with high grade and used without any further purification.
Instrumentation
Equipment used in this research were laboratory glassware and analytical mass balance (Mettler AT200). Infra-red spectra were obtained using Shimadzu-Prestige 21 spectrometer. 1H-NMR spectra were recorded at 400 MHz with a JEOL JNM-MY spectrometer using TMS as an internal reference. Chromatogram and mass spectra were measured on a Mariner LC-MS using C-18 column (15 x 1 mm) and injected volume of 2 µL. The mobile phase was methanol with flow rate of 0.1 mL/min.
Procedure
Synthesis of 1-monolaurin
Glycerol (0.01 mol, MW= 92.09 g/mol; 0.92 g), lauric acid (0.01 mol, MW= 200.03 g/mol; 2.003 g) and p-toluenesulfonic acid (pTSA) catalyst (2.5% w/w of lauric acid) were added in three-necked flask. The mixture was heated with continuous stirring at 130 °C for 6 h in oil bath. After that, crude product was cooled and dissolved in ethyl acetate. The solution was washed with distilled water until neutral pH. The organic layer was dried with Na2SO4 anhydrous and evaporated with rotary evaporator. The purification of
1-monolaurin from crude product (ethyl acetate layer) was conducted by using extraction with hydroalcoholic solution. The amount of hydroalcoholic solution (ethanol : water = 8 : 2) is 10 times of crude product (ethyl acetate layer) volume. The mixture in hydroalcoholic solution and the extracted 3 times with an amount of n-heksane. The optimization of reaction was carried out by using variation of pTSA catalyst of 5% and 7.5% w/w of lauric acid.FTIR (KBr) vmax:3487 (OH), 1736 (C=O), 1180 (C-O), 2924-2854 (Csp3-H), 1400 (-CH2-), 1300 (CH3) cm-1. 1H-NMR (400 MHz, CDCl3) δ: 0.86 (3H, t, CH3 of C12), 1.237-1.269 (16H, m, CH2 of C4-11), 1.573-1.646 (2H, m, CH2 of C3), 2.334 (2H, t, CH2 of C2), 3.590 (1H, dd, -CH2-(OH)CH–CH2-), 4.129 (1H, dd, -CH2-(OH)CH–CH2-), 3.590 (1H, dd, -CH2-(OH)CH–CH2-), 3.687and 4.191 (2H, dd, -(OH)CH–CH2-OCO-), 7.246 (1H, s, OH). 13C-NMR (400 MHz, CDCl3) δ: 14.217 (C12), 22.77 (C11), 24.991 (C10), 29.206 (C9), 29.330 (C8), 29.415 (C7), 29.673 (C8), 29.530 (C5), 29.673 (C4), 31.990 (C3), 34.220 (C2), 63.397 (-OH)CH2-(OH)CH-CH2-), 65.237 (-(OH)CH-CH2-OCO-), 70.338 (-(OH)CH-CH2-OCO-), 174.494 (C=O). LC-MS (100%). MS relative intensity (m/z) : 275,49 (base peak), 572 (2M+Na).
Antibacterial Assay
The composition of media for bacteria such as Nutrient Agar consisting of 5 g of peptone, 3 g of beef extract and 15 g of agar which were dissolved in 1 L of distilled water and then sterilized at a temperature of 121 °C for 15 minutes at 15 psi. On the surface of the petri dish, 50 mL of 1.8% agar solution was poured. After solidified, the wells are made by putting a ring with diameter of 1 cm and then 50 mL of media for pathogenic bacteria was poured in the surrounding that have been added with 1% culture of pathogens such as Staphylococcus aureus FNCC 0047 and Escherichia coli FNCC 0091. After solidified, as much as 150 µL of sample of 1-monolaurin was added by concentration of 500; 1,000; 2,500; 5,000 and 10,000 ppm with DMSO (99.9%) as negative control. The positive control of antibacterial assay is tetracycline with concentration of 500; 1,000; 2,500; 5,000 and 10,000 ppm while the negative control is distilled water. The dish was incubated for 24 h and the inhibitory zone was measured. A clear zone with size of more than 2 mm on the surrounding wells indicated as positive inhibition. The diameter of inhibition zone formed was measured by using a caliper to determine the effectiveness of antibacteria. Inhibition zone was measured by reducing the overall diameter (disk + inhibition zone) with diameter of disk.
Results and Discussion
Synthesis of 1-monolaurin through esterification of lauric acid and glycerol
1-Monolaurin compound could be synthesized from some pathways. This compound could be synthesized through esterification reaction of lauric acid and glycerol using acid catalyst. Widiyarti et al. (2009) synthesized 1-monolaurin through this reaction using H2SO4 catalyst with 31.05% yield of 1-monolaurin and 4.48% yield of dilaurin. The H2SO4 catalyst was predicted to be too strong and induced the formation of dilaurin so that reduced the yield of monolaurin. This reaction was conducted for 6 h at 130 °C with 5% (w/w) catalyst of total reactant. The synthesized monolaurin was also purified by column chromatography.
In the current paper, the synthesis of 1-monolaurin was conducted by reacting lauric acid and glycerol (1:1) with pTSA catalyst. The reaction was carried out at 130 °C for 6 h. The amount of pTSA catalyst was varied for 2.5%, 5.0% and 7.5%. The product was not purified by using column chromatography like the previous research. The esterification product was dissolved in ethyl acetate and extracted with base solution until the pH of organic layer is neutral. This step needs to be done because the pTSA catalyst is a heterogeneous catalyst and was separated from products by using base solution. The base solution will neutralized the catalyst to form a salt which is dissolved in water and easy to be separated. The ethyl acetate fraction was then evaporated and was dissolved in ethanol-water (80:20) solution or so-called hydroalcohol solution. The product was washed with n-hexane to afford a white solid. The yield of 1-monolaurin for 2.5%, 5.0% and 7.5% of pTSA variation was 60.34%, 43.54% and 27.89%, respectively. Thus, it can be seen that the highest percentage of yield of 1-monolaurin was the reaction with 2.5% of pTSA catalyst. The product was analyzed by using LC-MS and shown in Figure 1. Chromatogram of esterification product showed that 1-monolaurin was detected at retention time (tR) of 3.1 min. Single product was shown in the product of esterification using 2.5% and 5% of pTSA catalyst. Mass spectrum of product (Figure 2) showed fragment of M+H and 2M+Na with m/z of 275,49and 572 respectively. The elucidation of product by using FT-IR, MS, 1H-NMR and 13C-NMR was described in the procedure section.
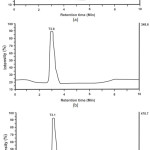 |
Figure 1: Chromatogram of esterification product using (a). 2.5%; (b) 5%; (c) 7.5% (w/w) of pTSA catalyst. Click here to View figure |
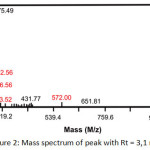 |
Figure 2: Mass spectrum of peak with Rt = 3,1 min Click here to View figure |
The purification of 1-monolaurin used the liquid-liquid extraction with hydro alcohol solution and n-hexane. Hydroalcoholic solution which contains of ethanol: water (80:20) could separate 1-monolaurin from the other non-polar impurities such as dilaurin, trilaurin and lauric acid residue. This purification technique is simpler and more effective compared to column chromatography technique from the previous research. The extraction technique using hydro alcohol solution could separate the 1-monolaurin until 100% of purity from the product of esterification using 2.5% and 5% of pTSA catalyst. The advantages of 1-monolaurin purification using liquid-liquid extraction are more effective in time and cost so that 1-monolaurin could be easily producedin a large scale.
Antibacterial assay of 1-monolaurin
1-Monolaurin has two hydroxyl and one lauryl groups that bound in glycerol scaffold. Lauryl groups as a non-polar group is bound at C1 of glycerol so that it called as
1-monolaurin (Figure 2). This compound was tested as antibacteria against S. aureus and E. coli. This assay used five of variation of sample concentration i.e. 500; 1,000; 2,500; 5,000; and 10,000 µg/mL and positive control of tetracycline with concentration of 500 µg/mL. The antibacterial assay used the same method as that of Widiyarti et al. (2009). The result of antibacterial assay was shown in Table 1
 |
Figure 3: Structure of 1-monolaurin |
Table 1: Diameter of inhibition zone of 1-monolaurin against S. aureus and E. coli
|
Bacteria |
Diameter of inhibition zone (mm) |
|||||
|
500 µg/mL |
1000 µg/mL |
2500 µg/mL |
5000 µg/mL |
10000 µg/mL |
Tetracycline 500 µg/mL |
|
|
S. aureus |
7,5 |
15.75 |
17.75 |
19.00 |
20,75 |
18.55 |
|
E. coli |
10.55 |
12.70 |
13.25 |
13.75 |
17.00 |
19.60 |
|
S. aureus * |
9.50 |
9.00 |
8.50 |
8.50 |
7.00 |
|
|
S. aureus** |
9.50 |
8.50 |
8.50 |
7.00 |
7.00 |
|
Negative control (DMSO): not inhibit the bacteria in all concentration
Antibacterial activity of 1-monolaurin synthesizedby Widiyarti et al. (2009)
Antibacterial activity of 1-monolaurin standard (Widiyartiet al., (2009)
The result showed that 1-monolaurin could inhibit the growth of S. aureusandE. coliin various concentration even at the lowest concentration of 1-monolaurin (500 µg/mL) although could not exceed the inhibition zone of tetracycline as positive control at the same concentration. The data showed that the higher concentration of 1-monolaurin will have the larger inhibition zone. Inhibition zone of synthesized 1-monolaurin against S. aureusandE. coliwas larger compared to those of 1-monolaurin standard and 1-monolaurin synthesized by Widiyarti et al. (2009). Thus, it can be concluded that the synthesized product have a good antibacterial activity for gram negative and gram positive bacteria.
Conclusion
1-Monolaurin was successfully synthesized by reacting lauric acid and glycerol (1:1) at 130 °C for 6 h using pTSA catalyst of 2.5%, 5% and 7.5% with 60.34%, 43.54% and 27.89% of yield, respectively. The synthesized 1-monolaurin could inhibit the growth of S. aureus and E. coli bacteria at 500 µg/mL of concentration.
Acknowledgements
Authors showed gratitude to Biosciences Laboratory at Universitas Nusa Cendana (Indonesia) for the equipment support to this research.
References
- Minister of Agricultural of Indonesia,Mengembalikan Kejayaan Kelapa Indonesia, Tabloid Sinar Tani Maret 2014, accessed22nd Juni 2014.
- Nitbani, F.O.; Jumina, Siswanta, D.; Sholikhah, E.N.;, Procedia Chemistry, 2016, 18, 132-140
CrossRef - Nitbani, F.O.;Jumina.; Siswanta, D.;Sholikhah, E.N; and Fitriastuti,D.; Orient. J. Chem.,2016,32(6), 1-8.
- Nitbani, F.O.; Jumina; Siswanta, D.; Sholikhah, E.N., Int. J. Pharm. Sci. Rev. Res., 2016, 39 (1), 74-80
- Freitas, L.; Paula, A.V.; dos Santos, J.C.; Zanin, G.M.; and de Castro, H.F.;J. Mol. Catal. B: Enzym., 2010, 65, 87–90.
CrossRef - Widiyarti, G.; Hanafi M.; and Soewarso W. P.;Indo. J. Chem., 2009, 9 (1), 99-106.
- Ugbogu, O. C.;Onyeagba, R. A.;and Chigbu, O. A.;Afr. J. Biotechnol., 2006,5(11), 1045-1047.
- Preuss, H.G.; Encard, B.; Enig, M.; Brook, I.; and Elliot, T.B.; Mol. Cell. Biochem.,2005, 272, 29–34
CrossRef - Wang, X.; Jina,Q.; Wang,T.; Huanga, J.; and Wanga, X.; J. Mol. Catal. B: Enzym.,2013, 97, 130-136
CrossRef - Pereira, C.C.B.; Silva, M.A.P.; and Langone, M.A.P.;J. Appl. Biochem. Biotech., 2004, 114, 433-446
CrossRef - Langone, M.A.P.; Dee Abreu, M.E.; Rezende, M.D.C.; and Sant’anna, G.L.JR.;Appl. Biochem. Biotechnol., 2002, Vols. 98–100, 987-996
CrossRef - Nitbani, F.O.; Jumina; Siswanta, D.; Sholikhah, E.N., Int. J. Pharm. Sci. Rev. Res., 2015, 35 (1), 126-136

This work is licensed under a Creative Commons Attribution 4.0 International License.









