Physicochemical Characteristics and Photocatalytic Activity of Silver Nanoparticles-decorated on Natural Halloysite (An aluminosilicate clay)
I. S. Fatimah and Rivaldo Herianto
and Rivaldo Herianto
Chemistry Department, Islamic University of Indonesia, kampus Terpadu UII, Jl. Kaliurang Km 14, Sleman, Yogyakarta, Indonesia.
Corresponding Author E-mail: isfatimah@uii.ac.id
DOI : http://dx.doi.org/10.13005/ojc/340232
Article Received on : January 06, 2018
Article Accepted on : February 25, 2018
Development of functional material for green chemistry applications become popular in recent years, and as part of these, preparation of nanoparticles for such application of organic contaminat is interesting topic. This research was aimed to prepare the chemical stable silver nanoparticles (AgNPs) for photocatalytic application over decorating onto natural clay mineral, halloysite. Prepared material was characterized using UV-visible spectroscopy, x-ray diffraction (XRD), Fourier Transform Infra-Red (FTIR), gas sorption analysis, scanning electron microscope (SEM) and transmission electron microscope (TEM) studies. From TEM analysis, it is found that AgNPs present in the diameter range of 20-50nm. The dispersed AgNPs slightly affect to increase the surface parameter of specific surface area and pore volume of the mineral. Photocatalytic activity test of the material exhibits that silver nanoparticle decorated halloysite shows excellent photocatalytic activity for photooxidation of rhodamine B.
KEYWORDS:Photocatalytic study; Silver nanoparticles-decorated; Natural halloysite
Download this article as:| Copy the following to cite this article: Fatimah I. S, Herianto R. Physicochemical Characteristics and Photocatalytic Activity of Silver Nanoparticles-decorated on Natural Halloysite (An aluminosilicate clay). Orient J Chem 2018;34(2). |
| Copy the following to cite this URL: Fatimah I. S, Herianto R. Physicochemical Characteristics and Photocatalytic Activity of Silver Nanoparticles-decorated on Natural Halloysite (An aluminosilicate clay). Orient J Chem 2018;34(2). Available from: http://www.orientjchem.org/?p=44817 |
Introduction
Silver is widely known as functional metal in wide applications such as antibacterial agent, optical functionalizing, electrical applications and medical and chemical sensors. The growing interests are related to synthesis of Ag in nanosize or called as silver nanoparticles (Ag NPs) as there is close relationship between the physical and chemical properties of nanomaterials for increasing activities in many applications. A material is categorized as nanoparticles as it has size of 1 – 100 nm. Basically nanoparticles synthesis can be conducted with top down (physics) and bottom up (chemistry) methods. Physics method is the manner of breaking metal solid into nano-sized small particles, meanwhile chemistry method is conducted by forming nanoparticles from molecular precusor or ionic [1][2].
Some techniques that can be used to produce nanoparticles are chemical, photochemical, sonochemical reduction, etc. Nanoparticles synthesis with sonochemical technique is using ultrasonic tool to break metal solid into nano sized particles, meanwhile photochemical technique is using highly UV radiation. There are many techniques can be used for producing nanoparticles such as i.e chemical, photochemical, sonochemical reduction, etc. For these scheme, Ag NPs were reported to be produced by green chemistry method such as utilizing plant extract as reductor for the synthesis[3][4]. In order to use Ag NPs for water treatment purposes, the trouble will be gained as AgNPs used as it form, moreover the reused Ag NPs will be a serious problem in treated water. Due to this limitation, another mechanism for increasing stability of AgNPs is supporting the nanoparticles onto a stable porous solid and usually called as decorating AgNPs. Some investigations reported the preparation of Ag NPs in composite forms with zeolite, clay, graphene oxide, silica and other polymer-solid composites[5–7]. Clay minerals are kind of solid having modifiable properties and also sufficient porous structure for supporting antibacterial activity and photocatalytic activity of Ag NPs. Shameli et al. (2010) prepared immobilized AgNPs within the interlayer of montmorillonite[8]. It is found that the nanoparticle size was about 20-30nm depend on the g-irradiation doses. By other varied parameters, Ahmad et al. (2009) showed that the particle size of nanoparticles is depending on the AgNO3 concentration. Using other kind of clay mineral, Pataklavi et al. (2003) and Hashemian and Shahedi (2013) utilized kaolinite mineral as support[9,10].
In general, the basic principle of Ag NPs synthesis was based on chemical reduction towards Ag+ before it anchored onto the solid support. Many researchers investigated the high potency of Ag NPs as photocatalyst dopant and photocatalyst itself. It is revealed that the combination of Ag with TiO2 in the composite form of kaolinite for example, the enhancement of dye degradation was obtained significantly. Beside on the potency of mineral clays for supporting photocatalytic activity of Ag NPs application, exploration on other kind of clay as support is an interesting topic for low cost photocatalysis.
Natural halloysite is one of phyllosilicate found in some places in Indonesia. Chemically, halloysite can be utilized for catalysis and functional material applications even until right now the utilization of halloysite in Indonesia is still related with ceramic production only. Refer to its potency for Ag NPs immobilizing solid, this research aimed to evaluate the preparation of Halloysite decorated with Ag NPs or furthermore called as AgNPs-Hal and its activity as photocatalyst.
Materials and Method
Silver nitrate (AgNO3, >99.9% purity), sodium borohydride (NaBH4,>99%purity), methylene blue, K2Cr2O7, H2O2, and rhodamine B were purchased Merck-Millipore, Germany. Natural haloysite (Hal) was obtained from Sukabumi, West Java, Indonesia.
Procedures
Preparation of AgNPs-Hal
Natural halloysite powder was dissolved with 250ml of water followed by stirring for 4 hours. a solution of 1×10-3M AgNO3 was added into the halloysite suspension and the stirring was kept for 1 h before the slow addition of NaBH4 0,1M. The mixture was then kept for overnight and the Ag NPs formation can be observed visually that it looks yellow to reddish. The mixture was filtered and the obtained powder was dried at the oven at 60oC for activity tests and characterization.
Characterization of materials (Halloysite and AgNPs-Hal) was performed by x-ray Diffraction (XRD), gas sorption analysis, Fourier-Transform Infra-red (FTIR) and Transmission Electron Microscope (TEM). XRD Shimadzu X6000, Quantachrome gas sorption analyzer, Perkin-Elmber FTIR and Transmission electrone microscope (TEM) of JEOL-JSM 6360 were employed for the analyses.
Photocatalytic Activity Test of AgNPs/Hal
Photocatalytic activity of prepared material was evaluated as photocatalyst in photo-oxidation of rhodamine B solution.The solution and photocatalyst was mixed in a photocatalytic reactor (Figure 1). For photooxidation process, H2O2 was added as oxidizing agent and 20 watt UV Lamp was employed as photon source in the photocatalytic mechanism. Reduction of rhodamine B concentration was calculated based on UV-Visible spectrophotometric analysis of treated solution by using following equation 1:

With Abst and Abso are absorbance of treated solution and the absorbance of initial solution, respectively.
Spectrophotometer HITACHI U-2010 was utilized for the analysis.
Evaluation of photooxidation kinetics and mechanism was also studied by chemical oxygen demand (COD) measurement. The COD value was determined by using reflux method of the samples with K2Cr2O7 followed by calculation based on spectrophotometric method.
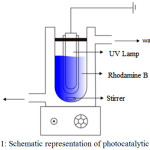 |
Figure 1: Schematic representation of photocatalytic reactor Click here to View figure |
Results and Discussion
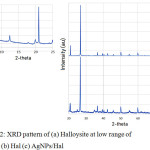 |
Figure 2: XRD pattern of (a) Halloysite at low range of 2-theta (b) Hal (c) AgNPs/Hal Click here to View figure |
Figure 2 shows the XRD patterns of Hal and AgNPs-Hal. Both materials demonstrate the peaks can be indexed to the characteristic peaks of halloysite refer to JCPDS No. 04-0783. The pattern of Halloysite shows the basal spacing reflections indicating a sharp peak at 12° (2q) which translate to a (001) basal spacing of 7 Å and there is no peak at 8.8° (2q) indicating the absence 10 Å form, which are indicative of hydrated halloysite. As shown by the patterns, it is confirmed that there is no significant different reflection peaks of both material assigning that there is no AgNPs patterns detected. This is assumed that the dispersed silver is in very low content with homogeneous distribution so there is no silver aggregate crystalline formed on surface.
The presence of Ag is proofed by SEM-EDX analysis (Fig. 3).
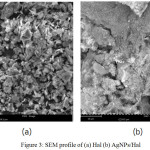 |
Figure 3: SEM profile of (a) Hal (b) AgNPs/Hal
|
From the morphology profile, it is seen that the dominant forms in Hal is flaky structure. The surface is changed after Ag NPs dispersion in that some dots appeared on surface. TEM analysis image (Fig. 4) reveals that the silver particles size are about 10-70nm in diameter.
The FT-IR spectra of the Hal and AgNPs/Hal were furthermore used to investigate the the resultant of functional groups of samples before and after AgNPs dispersion (Fig.5). The spectra at around 3400-3600 cm-1 are the indication for O-H vibration and found for both samples. Generally, the O-H refer to either silica-alumina structure or adsorbed H2O in the pores. The stretching spectra related to Al-O of halloysite structure are appeared in the range of 3620.47 cm-1 – 3696,07 cm-1 that are coincised with the spectra at around 1033.65 cm-1 and 913,48 cm-1 . The band at 1,032 cm‑1 is the indication of caused by the stretching vibration of Si-O-Si while the band observed at around 538 cm-1 was due to the vibration of Al-O-Si.
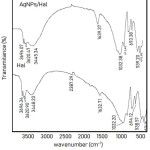 |
Figure 5: FTIR spectra of Hal and AgNPs/Hal
|
By comparing the spectra of Hal and AgNPs/Hal it is found that the are some shifts of the spectra at higher wavenumber are found for AgNPs/Hal i.e at around 1032 cm-1. The shifts may be related with the interaction between AgNPs and Halloysite structure case the higher energy required for the vibration.
The dispersion of AgNPs slightly affects to the surface profile as shown by adsorption-desorption pattern (Fig.6) and the calculated specific surface area, pore volume and pore radius are listed in Table 1. AgNPs/Hal exhibits the higher capability in adsorption as shown by the higher volume of adsorbed N2 in all range of P/Po compared to Hal. Due to the increasing adsorption-desorption profile the surface parameters of AgNPs/Hal suggest that the decorated AgNPs reflects the higher surface area form the dispersed particles.
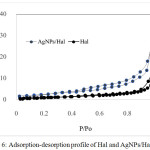 |
Figure 6: Adsorption-desorption profile of Hal and AgNPs/Hal
|
Table 1: Surface Parameters of AgNPs/Hal and Hal
| Paramaters | AgNPs/Hal | Hal |
| Specific surface area (m2/g) | 14.27 | 11.02 |
| Pore volume (cc/g) | 2.18 x10-3 | 1.94 x10-3 |
| Pore Radius (nm) | 1.49 | 1.51 |
Photocatalytic activity of AgNPs/Hal shows by kinetics of Rhodamine B photooxidation in Fig.7. The plots demonstrate the different kinetics of rhodamine B concentration along time of varied treatments: with the addition of AgNPs/Hal, the addition Hal with and without UV exposure. From the compared patterns, the rhodamine B reduction is obtained fastest by the treatment of the AgNPs/Hal addition with UV exposure while the kinetic profiles from other treatments are at similar values of C/Co in the same range of time. The patterns confirm the photocatalytic activity of AgNPs/Hal that is closely related with the presence of decorated-AgNPs since the Hal itself does not give photocatalytic activity[11]. Furthermore, the treatment without UV is also does not give excessive reduction, means that the oxidation occurs only over the presence of photon source from UV lamp.
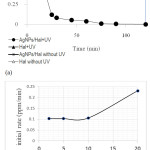 |
Figure 7: (a) Kinetics of rhodamine B photooxidation over varied treatments (b) Effect of initial concentration on initial rate of photooxidation
|
As shown in Fig. 7b, initial rate of rhodamine B oxidation is a function of initial concentration. The data is in line with general theory in that the concentration of reactant is the main factor for the reaction rate. As the oxidation process toward organic compounds, the photooxidation of rhodamine B is reflected by the reduction of chemical oxygen demand (COD) values after the treatment. The reduction of COD value indicates that rodamine B content in water is not only converted into other organic compounds but also removed (Fig.8).
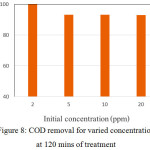 |
Figure 8: COD removal for varied concentration at 120 mins of treatment
|
Conclusion
Silver nanoparticles decorated halloysite is an alternative method for the synthesis of stable nanoparticles for photocatalytic purposes. Prepared silver nanoparticles decorated halloysite were characterized by using, XRD, FTIR, SEM and TEM techniques. From the studies, it is fond that the synthesized material showed the formation of silver nanoparticles at the range of 20-50nm on halloysite surface. The material demonstrated photocatalytic activity in rhodamine B photooxidation.
References
- C.A. Dos Santos, M.M. Seckler, A.P. Ingle, I. Gupta, S. Galdiero, M. Galdiero, et al., Silver nanoparticles: Therapeutical uses, toxicity, and safety issues, Journal of Pharmaceutical Sciences. 103 (2014) 1931–1944. doi:10.1002/jps.24001.
CrossRef - P. Sciau, Nanoparticles in Ancient Materials: The Metallic Lustre Decorations of Medieval Ceramics, in: Abbas A Hashim (Ed.), Nanotechnology and Nanomaterials » “The Delivery of Nanoparticles, InTech Open, 2012.
- A. Pal, S. Shah, S. Devi, Microwave-assisted synthesis of silver nanoparticles using ethanol as a reducing agent, Materials Chemistry and Physics. 114 (2009) 530–532. doi:10.1016/j.matchemphys.2008.11.056.
CrossRef - P. Jamdagni, P. Khatri, J.S. Rana, Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity, Journal of King Saud University – Science. (2016). doi:10.1016/j.jksus.2016.10.002.
CrossRef - W. Shao, X. Liu, H. Min, G. Dong, Q. Feng, S. Zuo, Preparation, Characterization, and Antibacterial Activity of Silver Nanoparticle-Decorated Graphene Oxide Nanocomposite, ACS Appl. Mater. Interfaces. 7 (2015) 6966–6973.
CrossRef - X.-W. Han, X.-Z. Meng, J. Zhang, J.-X. Wang, H.-F. Huang, X.-F. Zeng, et al., Ultrafast Synthesis of Silver Nanoparticle Decorated Graphene Oxide by a Rotating Packed Bed Reactor, Ind. Eng. Chem. Res. 55 (2016) 11622–11630.
CrossRef - B. Kaur, R. Srivastava, B. Satpatib, Silver nanoparticle decorated polyaniline–zeolite nanocomposite material based non-enzymatic electrochemical sensor for nanomolar detection of lindane, RSC Advance. 5 (2015) 57657–57665.
CrossRef - K. Shameli, M. Bin Ahmad, M. Zargar, W.M.Z.W. Yunus, A. Rustaiyan, N.A. Ibrahim, Synthesis of silver nanoparticles in montmorillonite and their antibacterial behavior., International Journal of Nanomedicine. 6 (2011) 581–590. doi:10.2147/IJN.S17112.
CrossRef - S. Hashemian, M. Reza Shahedi, Novel ag/kaolin nanocomposite as adsorbent for removal of acid cyanine 5r from aqueous solution, Journal of Chemistry. 2013 (2013). doi:10.1155/2013/285671.
CrossRef - R. Patakfalvi, I. Dékány, Incorporation of silver nanoparticles in kaolinite clays, Proceedings of SPIE – The International Society for Optical Engineering. 5118 (2003) 657–667. doi:10.1117/12.502000.
CrossRef - D. Latha, C. Arulvasu, P. Prabu, V. Narayanan, Photocatalytic Activity of Biosynthesized Silver Nanoparticle from Leaf Extract, (2017) 3–7. doi:10.2412/mmse.81.72.41.

This work is licensed under a Creative Commons Attribution 4.0 International License.









