Investigation of Different Ferrous Concentration Effect on Characteristics of NiFeP Nano Alloy Thin Films
1Research and Development Centre, Bharathiar University, Coimbatore-641 046, Tamil Nadu, India.
2Department of Physics, Jay shriram group of institutions, Avinashipalayam, Tirupur-638660,Tamil Nadu, India.
3PG and Research Department of Physics, Thiruvalluvar Govt. Arts College, Rasipuram-637 401, Namakkal, Tamil Nadu, India.
Corresponding Author E-mails: deviphd12@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340239
Article Received on : December 19, 2017
Article Accepted on : February 01, 2018
In this paper, electroplated NiFeP have been prepared and studied with different concentration of ferrous. The X-ray diffraction analysis demonstrates that the electro deposited NiFeP thin films consist of face centered cubic phase with diffraction peaks (220), (200) and (111). The EDAX analysis confirm that the maximum nickel content of 74.49 wt% was obtained for ferrous concentration 10 g/l. The weight percentage of ferrous increased while ferrous concentration was increased. The SEM result shows that NiFeP thin films were bright and uniformly coated on cathode surface. The Vicker Hardness Tester analysis shows that increases in hardness by increasing ferrous concentration. Also VSM studies shows decrease in coercivity and increase in magnetization were achieved by increasing ferrous concentration.
KEYWORDS:Electro Deposition; Characterization; Crystal; Magnetization; VSM; XRD; Micro Hardness; SEM
Download this article as:| Copy the following to cite this article: Devi C, Ashokkumar R. Investigation of Different Ferrous Concentration Effect on Characteristics of NiFeP Nano Alloy Thin Films. Orient J Chem 2018;34(2). |
| Copy the following to cite this URL: Devi C, Ashokkumar R. Investigation of Different Ferrous Concentration Effect on Characteristics of NiFeP Nano Alloy Thin Films. Orient J Chem 2018;34(2). Available from: http://www.orientjchem.org/?p=44616 |
Introduction
Thin film fabricated by magnetic materials have received much attention due to its potential applications in micro and nano electro mechanical systems .Nickel ferrous alloys are broadly used in electronic industries because of their inimitable structural and magnetic properties [1-3] . Nickel ferrous alloys are used as sensing materials in MEMS and NEMS for finding stress, magnetic flux and position. Also Ni-Fe alloy have been used in electronics, MEMS and NEMS devices such as laser diode, MOSFETs and sensing radiation. The reduction in thermal expansion of ferrous nickel alloys confirms mechanical stability with the ceramic substrates, semiconducting devices and glass windows on packaging. Magnetic materials can be deposited on the substrate using several methods [10-14]. Electro deposition is a important growth technique involving the materials deposition from an electrolyte bath by passing electric current at particular temperature and pressure. Important features of this method are cost-effectiveness, ability to deposit materials on substrates with complex geometries and easy control of the composition [15-19]. In addition, electro deposition allows morphology, composition and thickness of the deposited thin films by varying deposition parameters like temperature, current density, pH value and plating time [20-22]. The nickel ferrous alloy have been used in storage and recording devices in electronics, transformer with medium response sensitivity for residual current devices, pulse transformers, shielding for low temperature measurement and transducers [23-28].The use of ferrous with NiFe leads to good corrosion resistance, magnetic properties and structural properties of the alloy. In this study, the effects of different concentration of ferrous on NiFeP films were analysed. This paper explains the characterization of electrodeposited Ni-Fe-P alloy thin films with different concentration of ferrous.
Experimental Part
Electrodeposition of NiFeP alloy films were prepared with electrolyte baths consisting Ferrous sulphate (10,20,30,40 g/l), Nickel sulphate (50 g/l), Ammonium formate (40 g/l), Phosphorus Acid (10 g/l ), Citric acid (10 g/l) and operating at temperature (30 o C) .The solution pH was adjusted to 6.0 by adding NH4OH and electro deposition was carried out at current density 2 mA/cm2 and electro deposition time duration was 15 min. In this investigation, Cu and steel alloy substrates acted as cathode and anode respectively with dimension 7.5 cm x 1.5 cm [6-9]. After 15 minutes, the cathode plate was removed from deposition bath and dried for few minutes. The crystal phases and grain size of the electrodeposits were identified and analysed by the instrument X-ray diffractometer (XRD) with Cu K_ radiation. Morphological studies of Ni-Fe-P films were performed by the instrument Scanning Electron Microscope (SEM) and hardness of deposits was found by Vickers Hardness Test. The composition of Ni-Fe-P alloy films was found by EDAX (Energy dispersive X-ray Spectroscopy) and magnetic properties analyzed by the instrument vibrating sample magnetometer [29-30].
Results and Discussion
Elemental Composition of Nifep Thin Films
The elemental composition of NiFeP films was determined by EDAX analyser. The obtained data by this analyser are shown in Table 1. From result, the films with high concentration of ferrous sulphate exhibit high Phosphorus content. The high ferrous concentration of 41.38 wt% was obtained with ferrous sulphate concentration 40 g/l. Also nickel content decreases with increase of ferrous sulphate content. The high content of nickel 74.49 wt% was attained with lower ferrous concentration.
Table 1: EDAX analysis of thin films
| S. No | Ferrous Concentration(g/l) | FeWt% | NiWt% | PWt% |
| 1. | 10 | 18.15 | 74.49 | 07.36 |
| 2 | 20 | 21.62 | 69.73 | 08.65 |
| 3 | 30 | 29.03 | 58.14 | 12.83 |
| 4 | 40 | 41.38 | 44.19 | 14.43 |
Morphological Observation
Surface appearance of NiFeP thin films with increasing ferrous concentration were analysed by Scanning Electron Microscope (SEM) images and they are shown in Fig 1. The thin films are bright and uniformly coated on the surface. They are crack free by appearance.
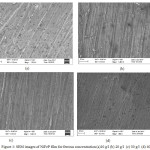 |
Figure 1: SEM images of NiFeP film for ferrous concentration (a)10 g/l (b) 20 g/l (c) 30 g/l (d) 40 g/l Click here to View figure |
Structural Characters
Structural characteristic (from XRD Data) results of deposited materials prepared with different ferrous concentration are shown in figure 2. From XRD pattern of FeNiP, crystal formation of deposits can be concluded. The size of crystals of can be determined by formula
Crystal Size (D) = (0.955 λ) / β Cos θ
Where, β is FWHM at 2θ, λ is wavelength of incident light. The XRD results of FeNiP films have shown face centred cubic phase with three diffraction peaks. The nano crystallite deposits was obtained and average crystallite size is around 29 nm.
 |
Figure 2: NiFeP alloy films- XRD patterns with different ferrous sulphate content (a) 10 gram/lit (b) 20 gram/lit (c) 30 gram/lit (d) 40 gram/lit |
The crystallite sizes of FeNiP deposits are tabulated in table 2.While ferrous sulphate concentration is increased from 10 to 40 gram/lit, the crystallite size decreases because of onset formation of crystal deposits during electro deposition process.
Table 2: NiFeP alloy films -Structural properties
| S.No | FerrousConcentration(g/l) | 2θ(deg) | d(A0) | Particle Size(D)(nm) | Strain(10-3) | Dislocation Density(1014 / m2) |
| 1 | 10 | 51.55 | 1.881 | 36.38 | 0.995 | 07.56 |
| 2 | 20 | 50.82 | 1.913 | 31.14 | 1.163 | 10.31 |
| 3 | 30 | 50.07 | 1.915 | 26.16 | 1.384 | 14.61 |
| 4 | 40 | 51.10 | 1.909 | 23.33 | 1.552 | 18.37 |
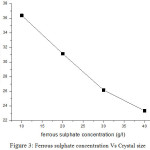 |
Figure 3: Ferrous sulphate concentration Vs Crystal size |
Mechanical Properties
Micro hardness measurement of deposits was done by Vickers hardness tester. The hardness values of thin films prepared with increasing ferrous concentration are 78, 107,149, 206 VHN respectively. Increasing value of hardness is concluded due to increases of ferrous sulphate content. This happen due to lower stress associated with thin films. Fig 4 shows the variation of hardness with increasing ferrous concentration.
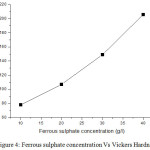 |
Figure 4: Ferrous sulphate concentration Vs Vickers Hardness |
Magnetic Characteristics
The important magnetic parameters were observed by vibrating sample magnetometer and shown in table 3.The hysteresis curves for Ni-Fe-P thin films for different ferrous concentration 10, 20, 30 and 40 gram/litre are shown in figure 5. It is noted that magnetization value increases from 0.0562 emu/cm2 to 0.3093 emu/cm2 when ferrous content increases from 10 to 40 gram/litre. So it is confirmed that the films obtained with higher ferrous concentration give higher magnetization values. An important character for Ni based alloy films is low coercivity. Crystallite size of film deposits decides the magnetization and coercivity. If the grain size of the deposits is big, the domain wall movement decide the magnetic characters of deposited materials. The magnetic characters can be improved by decreasing the crystal size of deposits. If crystal size is in order of nm, coercivity is reduced.
Table 3: Magnetic Parameters of NiFeP thin films
| S.No | Ferrous Concentration(g/l) | Coercivity Hs(G) | Magnetization Ms(emu/cm2) | Retentivity Mr(emu/cm2) | SquarenessS(Mr/ Ms) |
| 1 | 10 | 393 | 0.0562 | 0.0233 | 0.4145 |
| 2 | 20 | 277 | 0.0793 | 0.0254 | 0.3203 |
| 3 | 30 | 214 | 0.2470 | 0.1230 | 0.4979 |
| 4 | 40 | 129 | 0.3093 | 0.1241 | 0.4012 |
 |
Figure 5: NiFeP thin films – Magnetic hysteresis curves for ferrous concentration (a)10g/l (b) 20g/l (c) 30g/l (d) 40g/l |
The grain size of deposits is reduced by increase of ferrous concentration. Because of smaller crystalline size, Ni-Fe-P thin films obtained with ferrous concentration 40 g/l have high saturation magnetization and lower coercivity . From results, it is known that the best soft magnetic characters can be obtained with higher ferrous concentration.
Conclusion
In summary, an alloy thin films NiFeP have been prepared by electro deposition method. The characteristics of NiFeP films were observed. EDAX result has shown nickel content decreases with increase of ferrous sulphate content. The high nickel concentration of 74.49 wt% was obtained with lower ferrous concentration. The XRD results of FeNiP films have shown face centred cubic phase with three diffraction peaks and average crystallite size is around 29 nm. The thin films prepared with ferrous concentration 10 to 40 g/l are bright and uniformly coated on the surface. They are crack free by appearance. The hardness values of thin films prepared with increasing ferrous concentration are 78, 107,149, 206 VHN respectively. The saturation magnetization value increases from 0.0562 emu/cm2 to 0.3093 emu/cm2 and coercivity decreases when ferrous content increases from 10 to 40 gram/litre.
Acknowledgement
The authors wish to acknowledge Dr.T.Baskar, Professor, Department of Physics, Vidyaa Vikas College of Engineering and Technology, Tiruchengode, Tamilnadu, India for providing technical support.
References
- Dresselhaus, M.S.; Thomas, I.L.; Alternative energy technologies, Nature, 2001, 414332–337.
CrossRef - Di Bari, G.A.; Electrodeposition of nickel, Modern Electroplating, 2000, 5, 79-114.
- Gibbs, M.R.J.; Hill, E.W.; Wright, P.J.; Magnetic materials for MEMS applications, J. Phys. D: Appl. Phys, 2004,37, 237-244.
CrossRef - Brenner, A.; Electrodeposition of Alloys: Principles and Practice, vol. 1, 1963,Academic Press, New York.
- Ghorbani, M.; Dolati, A.; Afshar, A.; Electrodeposition of Ni–Fe Alloys in the Presence of Complexing Agents, Russ. J. Electrochem, 2002, 38, 1173-1177.
CrossRef - Sam, S.; Manavalan, G.; Guittoum ,A.; Gabouze, N.; Djebbar, S.; Electrodeposition of NiFe films on Si(100) substrate, Surf. Sci., 2007, 601, 4270 – 4273.
CrossRef - Dahms, H.; Croll, I.; The Anomalous Co deposition of Iron‐Nickel Alloys, J. Electrochem. Soc., 1965,112 , 771-775.
CrossRef - Barbano, E.P.; de Carvalho, M.F.; Carlos, I.A.; Electrodeposition and characterization of binary Fe-Mo alloys from trisodiumnitrilotriacetate bath, J. Electroanal. Chem., 2016,775 , 146-156.
CrossRef - Mik, A.; Hempelmann, R.; Lakatos-Vars_anyi, M.; _alm_an, E. K.; Iron-Phosphorous layers deposited by pulse electrochemical technique, Electrochem. Solid-State Lett., 2006, 9 (8) , 126-130.
CrossRef - Sam, S.; Manavalan, G.; Guittoum, A.; Gabouze, N. ; Djebbar, S.; Electrodeposition of NiFe films on Si(100) substrate, Surf. Sci. ,2007,601 , 4270 – 4273.
CrossRef - Esmaili, S. ; Bahrololoom, M.E.; Zamani, C.;Electrodeposition of NiFe/Cu multilayers from a single bath, Electron. Process. Mater. 2011, 47(2) , 10-15.
CrossRef - Yan ,X.H.; Sun ,J.Q.; Wang, Y.W; Yang, J.F.; A Fe-promoted Ni-P amorphous alloy catalyst (Ni-Fe-P) for liquid phase hydrogenation of m- and p chloro nitro benzene, Journal of Molecular Catalysis A: Chemical, 2006,252,17–22.
CrossRef - Gupta, N. ; Verma, A.; Kashyap, S.C.; Dielectric behavior of spin-deposited nanocrystalline nickel–zinc ferrite thin films processed by citrate-route, Solid State Commun. ,2005, 10 , 689-694.
CrossRef - An, Z.G.; Zhang, J.J.; Pan, S.L.; Fabrication of glass/Ni-Fe-P ternary alloy core/shell composite hollow Microspheres through a modified electroless plating process, Applied Surface Science, 2008, 255, 2219–2224.
CrossRef - Romig, A.D. ; Goldstein, J.; Determination of the Fe-Ni and Fe-Ni-P phase diagrams at low temperatures (700 to 300 C), Metall. Trans. A, 1980 , 11 , 1151-1159.
CrossRef - De Hosson, J.T.; Cavaleiro, A.; Galileo comes to the surface! in: Nanostructured coatings, Springer, 2006, pp. 1-26.
CrossRef - Motomura, Y. ; Tatsumi, T.; Urai, H. ; Aoyama, M.; Soft magnetic properties and heat stability for Fe/NiFe superlattices, IEEE Transactions on Magnetics ,1990, 26 , 2327-2331.
CrossRef - Dixit, G.; Singh, J.P.; Srivastava, R.C. ; Agrawal, H.M.; Choudhary, R.J.; Ajay, G.; Structural and magnetic behaviour of NiFe2O4 thin film grown by pulsed laser deposition, Indian J. Pure Appl. Phys., 2010,48 , 287-291.
- Tabakovic, I. ; Riemer, S. ; Vas’ko, V.; Sapozhnikov, V. ; Kief, M.; Effect of magnetic field on electrode reactions and properties of electrodeposited NiFe films. J. Electrochem. Soc., 2003, 150(9) , C635-C640.
CrossRef - Motomura, Y. ; Tatsumi, T.; Urai, H.; Aoyama, M.; Soft magnetic properties and heat stability for Fe/NiFe superlattices, IEEE Trans. Magn., 26 ,1990, 2327-2331.
CrossRef - Kuru, H.; Kockar, ; Alper, M.; Karaagac, O.; Growth of binary Ni–Fe films: Characterisations at low and high potential levels, J. Magn. Magn. Mater., 2015, 377 , 59-64.
CrossRef - Fernandez ,G.V; Grundy, P.J.; Vopson, M.M.; Control and analysis of grain Size in sputtered NiFe thin films, J. Phys, Condens. Matter, 2013, 1(1),6-9.
CrossRef - Celine, R.; Patrick, F.; Electrodeposition of thin films and nanowires Ni–Fe alloys, study of their magnetic susceptibility,J, Mater Sci., 2011, 46, 6046-6053.
CrossRef - Wang, S.L.; Studies of electroless plating of Ni-Fe-P alloys and the influences of some deposition parameters on the properties of the deposits, Surface and Coatings Technology, 2004, 186, 372–376.
CrossRef - Bedir, M.; Bakkaloglu, O.F.; Karahan, I.H.; Oztas, M.; A study on electrodepisted NixFe1-x alloy films, Pramana., 2006, 66(6) , 1093-1104.
CrossRef - Balachandran, R. ; Yow, H.K. ; Ong, B.H. ; Tan, K.B.; Anuar, K.; Wong, H.Y.; Surface morphology and electrical properties of pulse electrodeposition of NiFe films on copper substrates in ultrasonic field, Int. J. Electrochem. Sci. , 2011, 6, 3564-3579.
- Ghaferi, Z. ; Sharafi, S. ; Bahrololoom, M.E.; Effect of current density and bath composition on crystalline structure and magnetic properties of electrodeposited FeCoW alloy, Appl. Surf. Sci. 2015,355, 766-773.
CrossRef - Giouroudi, I.; Ktena, A.; Hristoforou, E. ; Microstructural characterization of cylindrical Fe1-xNix thin films. J. Optoelectron. Adv. M. 2004,6 661-666.
- Esmaili, S.; Bahrololoom, M.E.; Zamani, C.; Electrodeposition of NiFe/Cu multilayers from a single bath, Electron. Process. Mater., 2011, 47(2) 10-15.
CrossRef - Celine, R.; Patrick, F.; Electrodeposition of thin films and nanowires Ni–Fe alloys, study of their magnetic susceptibility, J, Mater Sci., 2011, 46, 6046-6053.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










