Synthesis and Characterization of Bio-Based Polyester and Polyamide from Citric Acid and Mannitol
Department of Polymer chemistry, School of Chemical Sciences, North Maharashtra University, Jalgaon-425001, (M.S.) India.
Correspoding Author E-mail: patilamardip007@rediffmail.com
DOI : http://dx.doi.org/10.13005/ojc/340161
The present work is synthesis of biobased polyesters from citric acid and mannitol while polyamide from citric acid and diethylene triamine (DETA). Both reactions are carried by melt condensation polymerization technique. The products are characterized by FTIR for functional group conversion. Also the acid value also determined. The thermal properties are characterized by thermo gravimetric analysis (TGA) and differential scanning calorimeter (DSC).
KEYWORDS:Biobased Polymer; Polyester; Polyamide; FTIR; TGA; DSC
Download this article as:| Copy the following to cite this article: Patil A. M. Synthesis and Characterization of Bio-Based Polyester and Polyamide from Citric Acid and Mannitol. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Patil A. M. Synthesis and Characterization of Bio-Based Polyester and Polyamide from Citric Acid and Mannitol. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=41495 |
Introduction
Recently the biobased polymers are attract the attention of scientist due to their biodegradability and biocompatibility. There are many biobased monomers which can be used for polymer preparation. Generally biobased monomers are easily available in abundance quantity and are economically cheap. Biobased compounds such as vegetable oil, citric acid, carbohydrates etc cane used for polymer synthesis. Beside the use of carbohydrate in food, biomass and raw materials they are modified with chemical reaction in applicable polymers. Many researcher are interested in carbohydrate polymer because it offering large hydroxyl functionality. Carbohydrates based polymers are being used in biomedicine, tissue engineering, polymeric detergents, macromolecular drugs, drug carrier and release systems, hydrogels, surface modifiers. [1-11]
There is a field of research in United state Department of Agriculture which study on the use of citric acid and carbohydrates and others have published reports of starch citrates, corn-fiber citrates, and cellulosic citrates. Citric acid was isolated from citrus fruits like lemon but recently fermentation method citric acid is produced commercially. Citric acid is easily available and economically cheap, in spite of increasing consumption in the food and beverage industries; also it is famous and used for its flavoring and buffering properties. It is also used in the petroleum industries as metal-chelating agent and cosmetics industries. Mannitol is natural sugar and has many industrial applications, medicinal uses as sugar it is often used as a sweetener in diabetic food as it is poorly absorbed from the intestines.
The present work is synthesis of biobased polymeric material from the citric acid and mannitol giving the polyester while polyamide from the reaction with citric acid with di-ethylene triamine (DETA). They are characterized by FTIR and their thermal properties are studied by TGA and DSC.
Experimental
Raw Materials
The following raw material was used in the synthesis; citric acid was obtained from s.d. fine India. The other chemicals mannitol, di-ethylene triamine and di-ethylene amine were purched loba chemie, India. The solvents ethanol and methanol were obtained from Rankem India. All the raw material used without purification.
Synthesis of Polyester from Citric Acid and Mannitol
A measured amount of mannitol (5.465g ) was weighted in 500 ml glass beake .Then desired amount of citric acid (1.921g) was added into it. The molar ratio is citric acid : mannitol is 1:3 maintained. This beaker of reaction mixture were placed inside the oven and polymerization is run at 150 to 1700C.There was no string during reaction, except that produced by bubbling (the release of water).Reaction completed after five hours remove the product for analysis.
Reaction Scheme 1
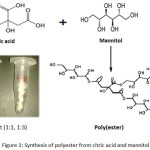 |
Figure 1: Synthesis of polyester from citric acid and mannitol Click here to View figure |
Synthesis of Polyamide from Citric Acid and Di-Ethylene Triamine (DETA) using Solvent
Procedure
The desired amount of citric acid (3.842gm, 0.02 moles) in 500ml glass beaker which is dissolved in minimum quantity of ethanol. Then measure di-ethylene triamine (3.095g, 0.03 moles).which added into the beaker drop wise manner with constant stirring. Reaction is carried out at room temperature and polymerization is formed with condensation. Then remove the product washes with solvent and dries it for characterizations.
Reaction scheme-2
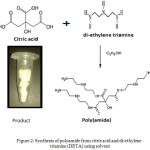 |
Figure 2: Synthesis of polyamide from citric acid and di-ethylene triamine (DETA) using solvent Click here to View figure |
Table 1: Formulation for Citric acid based polyester and polyamide
| Sr.No. | Citric Acid | Mannitol | Diethylene triamine (DETA) | Molar ratio |
| 1 | 1.921g | 1.8217 g | — | 1:01 |
| 2 | 1.921g | 5.465g | — | 1:03 |
| 3 | 3.842 g | — | 3.095 g | 2:03 |
Characterization
Determination of Acid Value of Polyester
Procedure
The weight 2 to 4 gram of sample dissolved in 50 ml of neutral methyl ethyl ketone (or any other water miscible suitable solvent) at room temperature. Then 2-3 drops of phenolphthalein indicator was added to the solution. Titratation of the solution against the standard alcoholic KOH solution until pale pink color appears permanently was done. Record volume of alkali consumed as an “A”ml. If the solvent is not neutral, carry out a blank titration and record the volume of alkali consumed as “B”ml.. Take at least 3 reading and then calculate the acid number. Acid value can be determined by using following formula.
![]()
IR Spectroscopy
IR of polyester and polyamide was determined on Perkin Elmer spectrum one FTIR spectrophotometer by pallet method using nujole oil.
TGA and DSC
Thermal analysis of polyester and polyamide was determined on Perkin Elmer TGA and DSC.
Results
Table 2: Acid values of polyester during reaction
| Sr. No. | Time | Acid value (mg of KOH/ gm of sample) |
| 1 | 10 min | 106.59 |
| 2 | 70 min | 98.175 |
| 3 | 130 min | 86.95 |
| 4 | 190 min | 75.735 |
| 5 | 220 min | 58.905 |
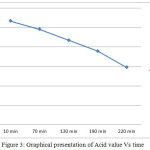 |
Figure 3: Graphical presentation of Acid value Vs time Click here to View figure |
From the above results it can be concluded that the acid value decreases with time. Prepared polyester was characterized in the laboratory by analysis
Acid Values for Solvent used Polyamide
In the initial state of reaction acid value is 173.91 and at the end of reaction acid value is decreases 56.1 mg of KOH/gm of sample.
IR of Polyester and Polyamide
IR for Poly (ester)
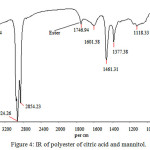 |
Figure 4: IR of polyester of citric acid and mannitol. |
IR of poly(amide)
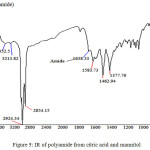 |
Figure 5: IR of polyamide from citric acid and mannitol Click here to View figure |
FTIR of the above polymer was taken using FTIR and data is analyzed as given below. FTIR spectra of our sample showed the important bands for presence of –OH group, -CONH2– group and esters groups also. Presence of other groups like –CH2– was also seen in the FTIR of the sample.
Table 3: IR frequencies of the polyester and amides
| Sr. No. | Frequency | Functional group |
| 1 | 2962 | C-H stretching |
| 2 | 1746 | Ester |
| 3 | 1746.94 | Ester stretching |
| 4 | 1638.33 | Amide |
| 5 | 1284 | CH2-CH2 stretching |
As FTIR contains frequencies for amide, ester and hydroxyl groups the products was polyester and polyamides it was as per the desire.
Thermal Gravimetric Analysis (TGA)
TG of Poly (ester)
This TGA data is showing the thermal stability of polymer. It shows two step degradation. First degradation occurs between 900 to 3050C, during that 40.936% of polymer is degraded. Second degradation start from 3050 to 5700C and during this temperature 48.689%polymer degraded.
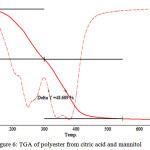 |
Figure 6: TGA of polyester from citric acid and mannitol Click here to View figure |
TG of Poly (Amide)
This TGA data is showing the thermal stability of polymer. It shows two step degradation. First degradation occurs between 1200 to 2800C, during that 43.762% of polymer is degraded. Second degradation start from 2800 to 4900C and during this temperature 32.728%polymer degraded.
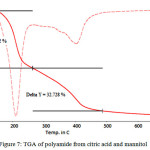 |
Figure 7: TGA of polyamide from citric acid and mannitol Click here to View figure |
Deferential Scanning Calorimeter (DSC)
DSC of poly (Ester)
This DSC data showing thermal stability. Polyester shoes glass transition temperature (Tg) is 22.950C and melting temperature is 133.830C.
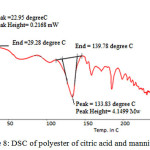 |
Figure 8: DSC of polyester of citric acid and mannitol Click here to View figure |
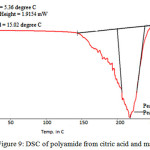 |
Figure 9: DSC of polyamide from citric acid and mannitol Click here to View figure |
DSC of poly (Amide)
This DSC data shows thermal stability. Polyamide shoes glass transition temperature (Tg) is 5.360 C and melting temperature is 218.340 C. it indicates thermal this polymer is very good.
Conclusions
We synthesized polyester using citric acid and mannitol with solvent free condensation polymerization method. The synthesis reaction occurs at both 150 and 1700 C, but it is significantly faster at the higher temperature. The molar ratio of the reactants is major factor in the material properties like solubility. The polyamide also synthesis with solvent and by solvent-free condensation polymerization method, using monomer citric acid and di-ethylene triamine. The synthesis reaction of solvent used polyamide occurs at room temperature and solvent free at 100 and 1100 C. These two polymers are novel and water soluble. Overall, this work has contributed to the understanding of the condensation polymerization of citric acid and mannitol bio based monomers and should help us shift towards the use of agricultural products in the biobased economy of the future.
References
- Doll, K.M.; Shogren, R. L.; Wilett, J. L.; Swipt, G. Journal of Polymer Science: Part A: Polymer Chemistry 2006, 44, 4259–4267.
CrossRef - Martel, B.; Ruffin, D.; Weltrowski, M.; Lekchiri, Y.; Morclletet, M.J Appl Polym Sci 2005, 97, 433-424.
CrossRef - Sikora, M.; Tomasik, P.; Pielichowski, K.Pol J Food Nutr Sci 1997, 6, 23-30.
- Wing, R. E. J Polym Mater 1997, 14, 303-309.
- Bellamay, L. The Infrared Spectra of Complex Molecules; Wiley; New York, 1958; pp 161-192.
- Wulff, G.; Schmid, J.; Venhoff ,T.; Macromol. Chem. Phys. 1996, 197, 259.
CrossRef - Choi, S.K.; Mammen, M.; Whitesides, G. M.; J. Am. Chem. Soc. 1997, 119, 4103.
CrossRef - Li, J.; Zacharek, S.; Chen, X.; Wang, J.; Zhang, W.; Janczuk, A.; Wang, P. G.; Bioorg. Med. Chem. 1999, 7, 1549.
CrossRef - Novick,S. J; Dordick, J. S; Chem. Mater. 1998, 10, 9.
CrossRef - Wulff, G; Zhu, L; Schmidt, H; Macromolecules,1997, 30, 4533.
CrossRef - Noordover, B. A. J.; Duchateau, R.; Van Bentham, A. T. M.; Ming, W.; Koning, C. E. Bio macromolecules 2007.
- Pramanick, D.; Bakr, M. A.; Ray, R. T.; J Polym Mater, 2002, 19,245-252.
- Jiang, M.; Chen,W; Fang,P; Chen,S; Bai,J; Huang,Y; Han,M; Lu,P; Dong, J; Journal of Polymer Science Part-A:Polymer Chemistry, 2012, 50, 3819–3829.
CrossRef - Aruna, P; Marie, M.; Iyer, P.; Int.J.Curr.Microbiol.App.Sci ,2014, 6, 255-261.
- Miuraj,Y.; Journal of Polymer Science: Part A: Polymer Chemistry, 2007, 45, 5031–5036.
CrossRef - Wulff,G.; Schmidt,H; Zhu,L; Macromol. Chem. Phys. 1999, 200, 774.
CrossRef

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.










