Synthesis and Study of Modified Polyvinyl Alcohol Containing Amino acid moieties as Anticancer Agent
Ali H. Samir , Ruwaidah S. Saeed and Fadhel S. Matty
, Ruwaidah S. Saeed and Fadhel S. Matty
Department of Chemistry, College of Education for Pure Science (Ibn Al-Haitham), University of Baghdad.
Corresponding Author E-mail: dr.mohammd08@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340131
A series of new phthalimides compounds were synthesized from reaction of Malic anhydride, phthalic anhydride, nitro phthalic anhydride, 2-phenyl-4H-benzo[d][1,3]oxazin-4-one, 2-(4-nitrophenyl)-4H-benzo[d][1,3]oxazin-4-one with different amino acids as glycine, alanine, valine, leucine, isoleucine, serine, threonine, tyrosine and Phenyl alanine [1]a-i under fusion conditions. Compounds react with SOCl2 in the presence of benzene to produce compounds. Chemical modification of Poly (vinyl alcohol) were obtained by reaction of PVA with compounds using the dimethyl formamide to give compounds. The structure of the synthesized compounds was characterized by their analytical and spectral data as, IR spectra,1H, C13-NMR, Elemental analysis (CHN), UV-Vis Spectroscopy, Scanning electron microscopy (SEM), Antibacterial activity were screened via two kinds of bacteria. Also, anticancer activity were examined for most of the modified polyvinyl alcohol.
KEYWORDS:Phthalimide; Polyvinyl Alcohol; Antibacterial and Anticancer Activities
Download this article as:| Copy the following to cite this article: Samir A. H, Saeed R. S, Matty F. S. Synthesis and Study of Modified Polyvinyl Alcohol Containing Amino acid moieties as Anticancer Agent. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Samir A. H, Saeed R. S, Matty F. S. Synthesis and Study of Modified Polyvinyl Alcohol Containing Amino acid moieties as Anticancer Agent. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=42139 |
Introduction
Cyclic imides and their derivatives brought much attention to chemist and pharmacist in the field of research and development(1), These compounds play an important role in medicinal chemistry in drug development and drug discovery(2). They Researches used these compounds as antibacterial(3), analgesic(4), nerve conduction blocking(5), hypotensive(6), muscle relaxant(7), antitumor(8)antitubercular agents(9)and antinociceptive agents,Also, these compounds interest as reactants for polymer synthesis(10).
In addition compounds containing phthalimide moiety are distinguished with antimicrobial (11-13), anti-inflammatory, anxiolytic, antiviral, antibacterial and antitumor properties (14,15).
Polyvinyl alcohol (PVA) is a water-soluble polyhydroxy polymer, non-halogenated aliphatic polymers, that has a two dimensional hydrogen-bonded network sheet structure(16).
PVA is a semi-crystalline polymer containing crystalline and amorphous phase(17) which is used in biomedical and pharmaceutical applications(18) and in industries due to the excellent chemical and physical properties, non-toxicity, good chemical resistance, good film formation capacity.(19)
It has been applied in production of many end products, as lacquers, resins, surgical threads, and food packaging materials (20).
Encouraged by these observation, the present study to synthesize new series of imide compounds containing amino acids with different heterocycles they may be have more activity and less toxicity as anticancer agents.
Aim of the present work is directed toward modification of polyvinyl alcohol containing active moiety with screened of antibacterial and anticancer activities.
Experimental
Materials
All the chemical used in the synthesis were supplied from BDH and Sigma-Aldrich.
nstrumentation
Melting points were recorded using electro thermal melting point apparatus and are uncorrected.
Infrared spectra were recorded as KBr disc on SHIMADZU-FT-IR-8400 spectrometer.1H,13C-NMR spectra was recorded on Bruker 500 MHz instrument using DMSO-d6 as a solvent and TMS as internal reference, measurement were made at Central lab ,Tahran University (Iran). the progress of the reaction was monitored by TLC using aluminum silica gel plates.
Synthesis of Compounds [2]a,b(21).
Benzoyl chloride or 4-nitrobenzoyl chloride(0.02mole) was added to a solution of 2-aminobenzoic acid (0.01mole) in (30ml.) pyridine .The mixture was shaken for 5 min and then kept in room temperature with shaking for 25 minute. Mixture was reacted with 15ml. 10% NaHCO3 , filtered , washed with water , dried and the crude product was recrystalized from absolute ethanol. The yield of compound[2]a was 81% , m.p (126) and [2]b was 77% , m.p (144).
General Procedure for Preparation of Compounds [3-7]a-i(22).
A mixture of equimolar amounts (0.001mole) of commercially available malic anhydride, phthalic anhydride, nitro phthalic anhydride , 2-phenyl-4H-benzo[d][1,3]oxazin-4-one, 2-(4-nitrophenyl)-4H-benzo[d] [1,3] oxazin-4-one were treated with corresponding amino acids[ 1]a-i in glacial acetic acid (15 ml.).
Mixture was refluxed for (5 hr.). A liquot of 25ml. of ice distilled water was added to the reaction. The compounds was filtered, dried and recrystallized from ethanol. The nomenclature and physical properties for prepared compounds
[3-7] a-i. were shown in Table (1)
Elemental analysis of compound[3]c
Calcd: C%=54.82 H%= 5.58 N% =7.10
Found: C%= 54.69 H%= 5.42 N% = 6.21
Elemental analysis of compound[5]i
Calcd: C%=60 H%= 3.52 N% =8.23
Found: C%=58.7 H%= 4.62 N% = 7.71
Synthesis of Compounds [8-12] a-i .(23)
A mixture of compound [3-7]a-i (0.01mole) and thionyl chloride (0.01mole) placed in dry benzene (10ml.) and refluxed for 7 hr . The excess of thionyl chloride and benzene were removed under vacuum after cooling .
Synthesis of Polymers [13-17] a-i(24)
(1mole) of PVA and (1mole) of compounds [8-12] a-i were placed in 20 ml DMF. The mixture was frequent shaking for 3hr. then refluxed for 2 hr., product was poured into the water, washed with a little sodium bicarbonate, washed with water, then with ethanol .The product purified by DMSO and reprecipitating from ethanol.
Biological Activity
Antibacterial Activity
Some of synthesized compounds have been screend for antibacterial activities against(Bacillus cereus and Esherichia coli) using cup-plate agar diffusion method(25). The zone of inhibition measured in mm. Pencilin was(50µg /ml) were used as a standard drug for antibacterial activity to compare with the activity of the synthesized compounds.
Cytotoxicity Assay
Preparation of Cell Lines for Cytotoxicity Assay:(26)
Fifteen modified PVA compound with different sizes and concentrations were screened for their anticancer activity and cytotoxicity by using cultured cells in microtiter plate (96wells). The assay was applied by the following steps:
Seeding: When cells in the incubated falcon became monolayer, the confluent monolayer was trypsinzed to get single cell suspension. A liquot 200 µl/104-105 cells/well from single cell suspension then were added to all the 96 wells of the microtiter plates, which covered by plate lids and sealed with adhesive parafilm. The plate was shaked gently and returned to the incubator.
Incubation: Microtiter Plates were then incubated in humidified chamber at 37°C, 5% CO2 until the cells reached confluence (i.e., vary according to the type of cell line). The plate was checked out for contamination , after cells attachment
Exposure: When the cells are in full of its activity, they were expose.d to three concentrations of the fifteen modified of PVA µg/ml for cell line. Aliquot of 200µl of each concentration were pipette into each well, while 200µl of maintenance medium were added to each well of control group , then plates were sealed with adhesive parrafilm and returned to the incubator. Evaluation of cytotoxicity was carried out after 48 hr. The photo picture were taken after 24 hr.
Staining: Cell viability was measured after 48 hr. of exposure by removing the medium, adding 20 µl/well solution of MTT and incubating for 4 hr. at 37°C. The crystals remaining in the wells were solubilized by the addition of 200 µl/well of (DMSO) followed by incubation in 37°C for 15 min with shaking. The absorbance was measured on a microplate reader at 620 nm. The rate of inhibition of cell growth was calculated according to(27) follow equation:
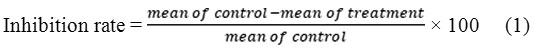
Results and Discussion
Scheme (1) summarized the performed reactions in this work. The structure of compounds [2]a,b were confirmed from its correct analytical and spectral data. FT-IR spectrum of compound [2]b , Figure (3.1), showed (21) appearance band at (1766) cm-1 due to the carbonyl group of cyclic ester, (1666,1614) cm-1 due to the C=N group and (1585) cm-1 due to the C=C group. The 1H-NMR spectrum of compound [2 ]b , Figure(3.2) display the following characteristic chemical shifts, (DMSO) ppm: the aromatic ring protons of compound [2]b appeared as multiple at δ (6.41-8.64) ppm.
N-phthaloyl amino acid derivative [3-7]a-i using economical experimental conditions via reaction Malic anhydride , phthalic anhydride, nitro phthalic anhydride, 2-phenyl-4H-benzo[d] [1,3] oxazin-4-one, 2-(4-nitrophenyl)-4H-benzo[d] [1,3] oxazin-4-one and different amino acids namely[1]a-i, glycine, alanine, valine, leucine , isoleucine, serine, threonine, tyrosine and Phenyl alanine in (15ml.) of glacial acetic acid , then mixture was refluxed for (5 hr.) . The mechanism (28) . involves nucleophilic addition reaction , as follows scheme (3.1).
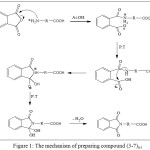 |
Figure 1: The mechanism of preparing compound (3-7)a-i Click here to View figure |
The structure of compounds [3-7]a-i was confirmed from its correct analytical and spectral data, FT-IR spectra of compounds [5]a,i , Figures [(3.3),(3.4)] , showed(22) bands at (3300-2400)cm-1 for (OH) of carboxylic acids, (1780,1735) cm-1 due to two (N-C=O), (1699) cm-1 for (C=O) of carboxylic acid .While1H-NMR spectrum of compound [5]a, Figure (3.5) , showed characteristic chemical shifts (DMSO-d6) ppm as follow : the aromatic ring protons appeared as multiple at δ (7.69-8.32) ppm and appearance singlet at δ (4.31) ppm due to CH2 protonand singlet in the region of δ 10.50 due to COOH proton.
The 1H-NMR spectrum of compound [5]i , Figure (3.6), display characteristic chemical shifts (DMSO-d6) ppm as follow: the aromatic ring protons appeared as multiple at δ (7.14-7.97) ppm and appearance doublet signal at δ (3.14) ppm related to CH2 proton and triplate signal at δ (3.99) due to CH proton.
The FT-IR spectrum of compounds[7]c , Figure (3.7) showed disappearance of due to the carbonyl group of cyclic ester at (1766)cm-1 and appearance band at (1685)cm-1 due to carbonyl group of carboxylic acid. Also, absorption bands at (1643)cm-1, (1608)cm-1 and (1587)cm-1 due to (C=O) of amide, (C=N) and (C=C) respectively . The1H-NMR spectrum of compound [7]c , Figure (3.8), showed characteristic chemical shifts (DMSO-d6) ppm as follow: a singlet signal at δ (12.36) ppm for proton COOH group, Many signals in the region δ (7.19-8.73) ppm that could be attributed to aromatic protons .Also appearance doublet signal at δ (4.13) ppm for proton CH-N group and many signals in the region δ (1.88) ppm that could be attributed to proton of CH in CH(CH3)2 and doublet signal at δ (0.96)ppm is due to (CH3)2 group .Whereas 13C-NMR spectrum of compound [7]c, Figure (3.9), showed : a signal at δ (172.91)ppm could be attributed to COOH group,while signal at δ (171.72)ppm is due to carbon of C=O amide group.Signal at δ (164.45) due to carbon of ph-C=N group. Many signal a δ (120-140)ppm could be attributed to carbon of benzene ring .Also signal at δ (59.1) ppm related to N-CH group. Signal appeared at δ (58.2) ppm is related to carbon of CH in CH (CH3)2.Two signal at δ (19.05-29.55)ppm could be attributed to(CH3)2.
N-phthaloyl amino acid chloride derivatives [8-12]a-i through h the reaction of N– phthaloyl amino acids [3-7]a-i with thionyl chloride in dry benzene was refluxed for(7 hr.) . A mechanism (29) for this reaction may be outlined as followed in scheme (3.2).
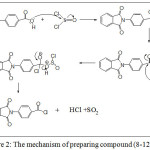 |
Figure 2: The mechanism of preparing compound (8-12)a-i Click here to View figure |
Compound [10]b was characterized by melting point and FT-IR spectrum . FT-IR spectrum of compound [10]b , Figure (3.10) , showed (23) the absence of absorption band at (1695) cm-1 and (3392) cm-1due to (carbonyl , hydroxyl) group of carboxylic acid and presence of band at (1761)cm-1 related to acyl chloride.
Chemical modification of Poly(vinyl alcohol)[13-17 ]a-i was obtained by reaction of PVA with compounds [8-12]a-i using the dimethyl formamide.
The compounds [13-17 ]a-i were identified by FT-IR spectrum . FT-IR spectrum of compound [15]a , Figure (3.11) illustrated the presence of a large peak at 3390cm−1this peak is related to the stretching of O–H from the intramolecular and intermolecular hydrogen bonds , which seen at 2908cm−1 and 2943 cm−1 respectively due to the symmetric and asymmetric stretching vibrational of C–H from alkyl groups (30), showed the disappearance of absorption band at (1761) cm-1 due to acyl chloride and appearance of absorption band at (1724) cm-1 due to carbonyl group of ester(31) and appearance of absorption bands at (C=O) of cyclic imide at (1710-1778) cm-1.
The 1H-NMR spectrum of compound [13]a, Figure(3.12), showed(32,33 ) the following characteristic chemical shifts (DMSO-d6) ppm showed the following signals: signal at δ(6.63) ppm for proton (CH=CH) group, singlet peak at δ(4.48) ppm for proton of(N-CH2)group, triplet peak at δ(4.24) ppm for (CH)group and doublet peak at δ(1.37) ppm for proton (CH2)group.
The 1H-NMR spectrum of compound [15]f, Figure(3.13), showed( 32,33) the following characteristic chemical shifts (DMSO-d6) ppm showed the following signals: many signals at δ (8.30-8.32) ppm for proton aromatic protons.triplet peak at δ(4.32-4.77) ppm for proton of(N-CH)group, doublet peak at δ(3.99) ppm for protonCH2 in (CH2-OH) , singelt peak at δ(3.45) ppm for proton (OH)group ,triplet peak at δ(3.05) ppm for (CH-CH2)group and doublet peak at δ(1.54) ppm for proton(CH2-CH)group.
The UV-Vis spectrum of compound [14]f , Figure(3.14)shows the absorption peaks at (332-402 ) may attributed to(π- π*) and (n-π*).
Table 1: FT-IR data of compounds[3-5]a-i
| Com. No. | (O-H)cm-1 | (C-H) arom.cm-1 | (C-H) aliph. cm-1 | (C=O) imide. cm-1 | (C=O) carboxlic |
| [3]a | 3400-2400 | 3053 | 2968-2879 | 1732-1770 | 1681 |
| [3]b | 3473 | 3072 | 2987-2873 | 1722-1784 | 1691 |
| [3]c | 3392 | 3066 | 2970-2890 | 1743-1782 | 1680 |
| [3]d | 3400-2400 | 3055 | 2965-2877 | 1728-1770 | 1690 |
| [3]e | 3464 | 3075 | 2939-2855 | 1716–1774 | 1691 |
| [3]f | 3462 | 3084 | 2939-2872 | 1749-1772 | 1697 |
| [3]g | 3442 | 3093 | 2920-2850 | 1745-1774 | 1693 |
| [3]h | 3400-2600 | 3051 | 2985-2939 | 1718-1735 | 1685 |
| [3]i | 3400-2400 | 3086 | 2972-2926 | 1722-1780 | 1680 |
| [4]a | 3344 | 3049 | 2989-2883 | 1734-1780 | 1683 |
| [4]b | 3400-2400 | 3080 | 2990-2951 | 1755-1786 | 1697 |
| [4]c | 3300-2400 | 3049 | 2966-2890 | 1712-1761 | 1691 |
| [4]d | 3400-2600 | 3109 | 3018-2990 | 1712-1764 | 1701 |
| [4]e | 3396 | 3090 | 2933-2877 | 1710-1772 | 1696 |
| [4]f | 3394 | 3089 | 2947-2885 | 1735-1776 | 1697 |
| [4]g | 3462 | 3084 | 2939-2872 | 1749-1772 | 1697 |
| [4]h | 3435 | 3088 | 2962-2893 | 1716-1772 | 1685 |
| [4]i | 3392 | 3045 | 2980-2943 | 1710-1770 | 1662 |
| [5]b | 3392 | 3089 | 2995-2941 | 1724-1784 | 1695 |
| [5]c | 3408 | 3041 | 2970-2881 | 1726-1784 | 1690 |
| [5]d | 3400-2400 | 3115 | 2960-2860 | 1732-1782 | 1683 |
| [5]e | 3400-2400 | 3064 | 2926-2854 | 1716-1780 | 1683 |
| [5]f | 3398 | 3024 | 2990-2889 | 1732-1776 | 1681 |
| [5]g | 3408 | 3097 | 3039-2926 | 1716-1780 | 1690 |
| [5]h | 3400-2600 | 3026 | 2929-2856 | 1716-1734 | 1701 |
Table 2: FT-IR data of compounds[6,7]a-i
| Com. No. | (O-H)cm-1 | (C-H) arom.cm-1 | (C-H) aliph. cm-1 | (C=O) carboxlic | (C=O) amid.cm-1 |
| [6]a | 3442 | 3049 | 2943-2895 | 1685 | 1643 |
| [6]b | 3400-2400 | 3043 | 2989-2933 | 1683 | 1643 |
| [6]c | 3442 | 3061 | 2966-2879 | 1680 | 1645 |
| [6]d | 3433 | 3024 | 2968-2889 | 1697 | 1676 |
| [6]e | 3305 | 3064 | 2966-2877 | 1683 | 1645 |
| [6]f | 3400-2600 | 3062 | 3030-2951 | 1683 | 1668 |
| [6]g | 3400-2400 | 3064 | 2960-2883 | 1685 | 1643 |
| [6]h | 3300-2600 | 3051 | 2960-2887 | 1685 | 1643 |
| [6]i | 3400-2600 | 3062 | 2926-2858 | 1690 | 1654 |
| [7]a | 3502 | 3078 | 2965-2879 | 1681 | 1645 |
| [7]b | 3400-2400 | 3082 | 2985-2858 | 1693 | 1655 |
| [7]d | 3400-2600 | 3059 | 2999-2854 | 1689 | 1645 |
| [7]e | 3508 | 3057 | 2962-2875 | 1681 | 1640 |
| [7]f | 3498 | 3080 | 2924-2850 | 1689 | 1655 |
| [7]g | 3508 | 3061 | 2985-2856 | 1681 | 1647 |
| [7]h | 3300-2400 | 3007 | 2929-2827 | 1690 | 1666 |
| [7]i | 3508 | 3061 | 2929–2856 | 1681 | 1668 |
Biological Activity
Antibacterial Activity
All the newly synthesized derivatives were screened for their in vitro antimicrobial activity against Escherichia coli, Bacillus cereus by measuring the zone of inhibition in mm. Result showed that compounds[6]f and[16]f exhibit some antibacterial activity with penciline against E .coli while compounds [15]f and [17]f showed antibacterial activity closed to penciline against Bacillus cereus. Resalts of all compounds all compounds and their antibacterial activities listed in Table (3.4).
Table 3: The inhibition zone of some synthesized compounds.
|
Compound |
E .coli -mm |
Bacillus cereus mm |
|
Penciline |
16 |
22 |
|
DMSO |
Nil |
Nil |
|
[3]h |
10 |
10 |
|
[13]h |
15 |
16 |
|
[4]h |
10 |
10 |
|
[14]h |
10 |
21 |
|
[5]f |
15 |
16 |
|
[15]f |
15 |
23 |
|
[6]f |
16 |
13 |
|
[16]f |
16 |
21 |
|
[7]f |
14 |
18 |
|
[17]f |
14 |
25 |
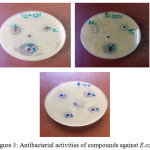 |
Figure 3: Antibacterial activities of compounds against E.coli Click here to View figure |
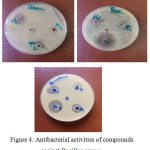 |
Figure 4: Antibacterial activities of compounds against Bacillus cereus. Click here to View figure |
Anticancer Activity
Fifteen compounds modified polyvinyl alcohol were selected for examend their anticancer activity in Bio-technology research center, Al-Nahrain University, Baghdad, Iraq. Two cell lines were used (mice intestines carcinoma cell line L20b and human pelvic rhabdomyosarcoma (RD) according to the method described by Freshney(26). Results are expressed in percentage. All compounds except [17]b and [17]d showed more than 50% inhibition for mice intestines carcinoma cell line , while these compounds[17]b and [17]d exhibit inhibition more than 50% inhibition for human pelvic rhabdomyosarcoma.
Table 4: The inhibition of cells growth of some synthesized compounds. µl/well
| Compound |
inhibition of cells growth for L20b |
inhibition of cells growth for RD |
|
[13]c |
53.7% |
51.1% |
|
[13]e |
69% |
47.5 % |
|
[14]c |
54.3% |
44.6% |
|
[14]e |
58.3% |
25.5% |
|
[14]i |
60.4% |
55.1% |
|
[15]h |
74.81% |
71.9% |
|
[15]i |
70.% |
69.2% |
|
[16]b |
63.7% |
60.4% |
|
[16]h |
67% |
49.8% |
|
[16]e |
50.2% |
35.8% |
|
[16]i |
56% |
49.5% |
|
[17]b |
42.4% |
50.1% |
|
[17]d |
35.3% |
56.3% |
|
[16]f |
51.5% |
36.9% |
|
[16]g |
56.% |
50..9% |
 |
Figure 5: Image of before well Staining Click here to View figure |
 |
Figure 6: Image of well after Staining Click here to View figure |
 |
Figure 7: Image of plate before Staining Click here to View figure |
 |
Figure 8: Image of plate after Staining Click here to View figure |
![Figure 9: SEM of compound[9]f](http://www.orientjchem.org/wp-content/uploads/2018/01/Vol34No1_Nov_Ali_fig91-150x150.jpg) |
Figure 9: SEM of compound[9]f Click here to View figure |
![Figure 10: SEM of compound[16]h](http://www.orientjchem.org/wp-content/uploads/2018/01/Vol34No1_Nov_Ali_fig10-150x150.jpg) |
Figure 10: SEM of compound[16]h Click here to View figure |
Conclusion
Compounds react with SOCl2 in the presence of benzene to produce compounds. Chemical modification of Poly(vinyl alcohol)were obtained by reaction of PVA with compounds using the dimethyl formamide to give compounds. The structure of the synthesized compounds was characterized by their analytical and spectral data as, IR spectra,1H, C13-NMR, Elemental analysis (CHN), UV-Vis Spectroscopy, Scanning electron microscopy (SEM), Antibacterial activity were screened via two kinds of bacteria. Also, anticancer activity were examined for most of the modified polyvinyl alcohol.
Acknowledgments
I would like to thank the University of Baghdad- Faculty of Education (Ibn-Al-Haitham) Department of Chemistry to help me for complete this article.
Refrences
- S S. Rajput and R.A. Mohammed Ali Sayyed .Synthesis, characterization and Biological Evaluation of 3,4-bis(substituted-Phenyl)-7-(2,6- dichloro-4-(trifluoromethyl) phenyl) -7H-pyrrolo [2,3-C: 5,4- C’] diisoxazoles from2, 6- dichloro-4-trifluoro methyl aniline. Shankarsing Sardarsing Rajput , IJCTPR, 2017, ISSN: 2321-3760 , 5(1): 17–22 .
- A. H. Samir, I. y. Majeed and S. M. Hasan, Ibn-Al-haitham J. for Pure & Appl. Sci. 2014, 27(3), 407-420.
- S S. Rajput and R M. Sayyed. Synthesis and formylation of cyclic imides using Vilsmeire- Haack reaction from 2, 6 dichloro-4- triflouromethyl aniline and their anti-microbial activity. World Journal of Pharmaceutical Research, 2015, 12: 1689-1695.
- M M .Patil and S S. Rajput. Succinimides synthesis and biological activity. International Journal of Pharmacy and Pharmaceutical Sciences, 2014,11:0975-1491.
- R S. Dhivare and S S. Rajput. Synthesis and antimicrobial evauation of some novel bisheterocyclic chalcones from cyclic imides under microwave irradiation.Chemical Science Review and letter, 2015, 4(15): 937-944.
- F C Pennington, P A Guercio and I A.Solomons. The antihypertensive effect of a selective central muscarinic cholinergic antagonist: N-(4- diethyl amino -2-butynyl)-succinimide. J. Am Chem Soc, 1953, 75(9): 2261-1.
CrossRef - D L Musso, F R Cochran, J L Kelley, E W McLean, J L Selph and G C Rigdon, et al. Design and synthesis of (E)-2-(4,6-Difluoro-1- indanylidene) acetamide , a potent, centrally acting muscle relaxant with anti- inflammatory and analgesic activity.J Med Chem, 2003,46(3): 399-408.
CrossRef - J H Mansoory and S S Rajput. Synthesis, characterization and biological evaluation of some novel Schiff’s bases from Halovinyl aldehyde and 4- amino-5(pyridine-4-yl)-4H-1, 2, 4-triazole-3-thiol.Der Pharma Chemica, 2015, 7(10): 510-514.
- M. Isaka, W. Prathumpai, P. Wongsa, M. Tanticharoen and Hirsutellone F. A dimer of antitubercular alkaloids from the seed fungus Trichoderma species BCC 7579. Org Lett, 2006, 8(13):2815-7.
CrossRef - A. M. Al-Azzawi and A. S. Hamd ,synthesis, characterization and antimicrobial activity evaluation of new cyclic imides containing 1,3,4-thiadiazole and 1,3,4- oxadiazole moieties. International journal of research in pharmacy and chemistry (IJRPC)2013,ISSN: 2231-2781, 3(4).
- G S Gruzdyes, V A Zincchenko, R I Slovtsov. The Chemical Protection of Plants, by Gruzdyes GS. Mir Publishers, Moscow. 1983;272.
- Y L Nene, P N Thapliyal. Fungicides In Plant Disease Control, 3th Ed., Oxford & IBH Publishing Co. Pvt. Ltd., New Delhi. 1993;170. .
- U S Ramulu Sree. Chemistry of Insecticides & Fungicides, 2nd Ed., Oxford & IBH Publishing Co. Pvt. Ltd., New Delhi. 1995;246.
- Ullmanns. Encyclopedia of Industrial Chemistry, 5th Ed., Vol. A 20, Editors Elvers B, Hawkins S, Sehulz G. 1991;190.
- OMO Habib , EB Moawad , SD Badawy, JAF Mansour. Some New Heterocyclic Sulphonates with potential antimicrobial activity: J. Prakt. Chem. 1990;332:791. Skehan, P.; Storeng R, Scudiero. D. J. Natl, Cancer Ins. 1990;82 :1107.
CrossRef - O. W. Guirguis, and M.T. H. Moselhey. Thermal and structural studies of poly(vinyl alcohol) and hydroxypropyl cellulose blendsNatural Science (2012) Vol.4, No.1, 57-67.
- A.M.Shehap and Dana S.Akil. Structural and optical properties of TiO2 nanoparticles/PVA for different composites thin films. Int. J. Nanoelectronics and Materials 9 (2016) 17-36 .
- SN Zadeh , S Rajabnezhad , Zandkarimi M , S Dahmardeh, Mir L, et al. Mucoadhesive Microspheres of Chitosan and Polyvinyl Alcohol as A Carrier for Intranasal Delivery of Insulin: In Vitro and In Vivo Studies. (2017) .MOJ Bioequiv Availab 3(2): 00030. DOI: 10.15406/mojbb.2017.03.00030
CrossRef - A.Samzadeh-Kermani,M.Mirzaee and Mansour Ghaffari-Moghaddam . Polyvinyl Alcohol/Polyaniline/ZnO Nanocomposite: Synthesis, Property. Advances in Biological Characterization and Bactericidal Chemistry, 2016, 6, 1-11.
CrossRef - T. Sumer Gaaz , Abu Bakar Sulong , Majid Niaz Akhtar , Abdul Amir H. Kadhum , Abu Bakar Mohamad and Ahmed A. Al-Amiery Properties and Applications of Polyvinyl Alcohol, Halloysite Nanotubes and Their Nanocomposites.Molecules2015,20,22833–22847.
CrossRef - G.Deepa , P.Pantel , M.shah ; P.S.Patel ; Der pharma chemia (2012); 4 (2)pp.626-628.
- YA .Nagiev , J .Russiam. Organic chemistry , 2012,48(3)pp.469-472.
- M.S Fouad , I .Redha Al-Bagati ;A. Al –Juboori ;Al-Mustansiriya J.Sci , 2006,17(3),pp.15-26
- L. S.Ahamed. Synthesis of New Polyester-Amides from Polyvinyl Alcohol and Convert Some of Them to Polyester-Imide, Journal of Al-Nahrain University 2011, June,Vol.14 (2) , pp.29-42.
CrossRef - A.L Barry, The Antimiccrobial Susceptibility Test : principle and practices ,(Len and Febiger ,Philadelphia ,USA), (1977), 180; Biol Abstr , 64 ,25183 .
- RI. Freshney, “Culture of Animal Cells: A manual of Basic Technique and Specialized Applications,” 6th Edition, Wiley: New York, 2010.
CrossRef - S. Gao .et al,“Antiproliferative effect of octreotide on gastric cancer cells mediated by inhibition of Akt/PKB and telomerase,” World J. Gastroenterol., Vol. 2003,9 (10), p.p.2362- 2365.
- E.T. Ali ;K.M.Lazim AL-Aliawy and J.H.Tomma .IBN AL-Hatham J. For pure and Appl .Sci . 2011,24(3).
- Solomons and fryhle “organic chemistry main and advanced” maestro A wiley Brand (2013).
- H. Awada and C. Daneault , Chemical Modification of Poly(Vinyl Alcohol) in Water Appl. Sci. 2015, 5, 840-850 .
- E. G. Crispim, J. F. Piai1, A. R. Fajardo, E. R. F. Ramos, T. U. Nakamura, C. V. Nakamura, A. F. Rubira , E. C. Muniz, eXPRESS Polymer Letters (2012),Vol.6, No.5 .383–395.2012, 2(5): 79-84 .
- Smith J.G.,Organic Chemistry , 1st ed , MC Graw Hill , New York , 2006,522.
- Roman Jantas, Zbigniew Draczyński, Lucyna Herczyńska , Dawid Stawski , Poly(vinyl alcohol)-Salicylic Acid Conjugate: Synthesis and Characterization ,American Journal of Polymer Science 2012, 2(5): 79-84 DOI: 10.5923/j.ajps.20120205.01.
CrossRef

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









