Synthesis of A Novel PE-3030 Based WPUA Hybrid Binding Substance for Pigment Printing
Sudip Kumar Lahiri1, Md Kamruzzaman1, Md Eman Talukder1, Md Nahid Pervez1, 2 and Quan Heng1
1Wuhan Textile University, School of Chemistry and Chemical Engineering, Wuhan 430200, P.R, China.
2Research Institute of Flexible Materials, School of Textiles and Design, Heriot-Watt University, Galashiels TD1 3HF, UK.
Corresponding Author E-mail: nahid.tex92@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330501
In this research, the waterborne polyurethane acrylate (WPUA) hybrid emulsions were first fabricated based on polyester polyol (PE-3030) and acrylic monomers via emulsion polymerization. The structures and thermal properties of prepared WPUAs were characterized by FT-IR and TGA. Then, physical properties of WPUAs i.e., average particle size, stability, viscosity; surface tension and solid content were determined. Some mechanical performances and solvent resistance of emulsions were also examined. Fabric screen printing application of the pigment-based inks formulated with the as-considered produced emulsion as a binder demonstrated good colour strength, fastnesses and handle of the printed fabrics compared to commercial binder. In addition, shows a significant improvement in tear strength and crease recovery angle properties. Particularly, PE-WPUA-10 sample was considered best due to exhibited superior performance in all properties. Synthesized polyester based WPUA is considered to be a future waterproof adhesive to fulfil the use standards for consumers.
KEYWORDS:PE-3030; Emulsion Polymerization; WPUA; Binder; Surface Tension
Download this article as:| Copy the following to cite this article: Lahiri S. K, Kamruzzaman M, Talukder E. M, Pervez N. M, Heng Q. Synthesis of A Novel PE-3030 Based WPUA Hybrid Binding Substance for Pigment Printing. Orient J Chem 2017;33(5). |
| Copy the following to cite this URL: Lahiri S. K, Kamruzzaman M, Talukder E, Pervez N, Heng Q. Synthesis of A Novel PE-3030 Based WPUA Hybrid Binding Substance for Pigment Printing. Orient J Chem 2017;33(5). Available from: http://www.orientjchem.org/?p=37724 |
Introduction
Green chemistry, relate inescapable pattern of industry advancement, is that the forward position within the chemistry field at present era. In polymer chemistry, by reducing the practice of organic solvent during theprocess of synthesis is anidealstep for creating ecological manner. Due to this scenario, waterborne polyurethane (WPU) received a remarkable interest for packages in coatings, clinical gadgets, sealants and the textile sector, great attachment on substrates, elasticity, abrasion resistance and zero/low volatile organic compounds (VOCs) [1-4]. Moreover, the hardness and versatility of these films must be proportioned by using of selected various the constitutions of hard and flexible segments [5]. The polyurethane (PU) synthesis is composed of polyaddition reaction between the hydroxyl group (–OH) of polyol and the isocyanate group (–NCO) [6]. The most responsible part of PU is made out of a polyol (soft segment), a diisocyanate (hard segment) and a low-molecular-weight diol component (chain extender, hard segment). A notable element of PU is polyol which presents flexible segment, cross-linking site and additionally offer the thermosetting ability to the ensuing polymers. There are different types of polyols (hydroxyl terminated polymers), polyesters, acrylics, polyethers and renewable source based polyols available [7]. Nonetheless, polyester polyol based emulsion is the most available polyol dispersion component. The influence of polyester polyol structure in dispersion component provides a substantial effect on WPUproperties, such as adhesion, hardness and impact resistance. The intensified product storage stability is tremendously preferred in many used packages. The polyester polyol dispersion is known for superior storage stability due to high resistance ester bond [8], but there are a lot of easily hydrolyzed ester bond in polyester polyol dispersion [9] and this reduces film properties and storage stability of polyester polyol dispersion. However, they have some drawbacks, such as feeble water resistance due to the hydrophilic group in their molecule chains and limited outdoor durability, which restrict their extent of uses in specific perspectives [10]. To get the best adjust of properties at a sensible cost, the combination of PU with a low cost material is now a common recurring inside the coating marketplace [11]. Acrylic polymers (AC) emulsions have bring down cost, phenomenal water resistance and mechanical properties because of the fundamental polymer chain’s carbon-carbon bond, which are likewise the most essential binders in the same application [12]. However, the elasticity and abrasion resistance of their films are inferior to those of PU films. A technique to attain the great residences of each structure is to synthesize the waterborne polyurethane/polyacrylate (WPUA) composite emulsion particles. In the system, the PU dispersion is considered as seed emulsion for ensuing emulsion polymerization. However, long drying time, transportation costs, storage costs, and shelf-life are relatedconcern of WPU emulsion. These related matters are addressed with high solid content and smaller particle size that makes their applications more efficient with longer shelf-life, reduction in cost, time, and energy loads [13]. Therefore, the combination of PU and AC to prepare waterborne polyurethane/acrylic (WPUA) hybrid emulsions has attracted a well-known interest both in academia and in industry. Such type of waterborne polyurethane acrylate (WPUA) emulsions are anticipated to offer the advantages of each issues, such as excellent weather resistance, affinity to pigments, lower cost, better mechanical stability, excellent adhesion, abrasion resistance, solvent, and chemical resistance [14, 15].
Intrigued by PU adjustments, the novelty of this work is, the synthesis and utilization of a new kind of PE-3030 based WPUA hybrid emulsion with high performance through emulsion polymerization for textile application which has not done before. To establish the chemistry and to evaluate the efficacy range, emulsions have been subjected to important tests and characterised by using distinct state-of-the-art techniques were located.
Experimental
Materials
100% cotton scoured woven fabric having construction 26 ends/cm2, 24 picks/cm2 and an area density of 152 gm/m2 was supplied by local textile Mill and used for pigment printing purpose. Polyol (PE -3030)was supplied by Zhejiang Huafeng New Materials Corporation Ltd. Isophorone diisocyanate (IPDI) was supplied by Sinopharm Chemical Reagent Co., Ltd. Acetone, 1,4-butanediol (BDO), DBTDL, Hydroxyethyl methacrylate (HEMA), and triethyl amine (TEA) were supplied by Tianjin Guangfu Fine Chemical Research Institute. Dimethylolpropionic acid (DMPA)was dried in a vacuum oven at 80oC for 24 h before use, Sodium dodecyl sulfate (SDS) and Fatty alcohol polyoxyethylene ether (AEO9) were received from Tianjin Chemical Reagent Factory. Acrylic acid (AA), methacrylic acid (MAA), butyl acrylate (BA), styrene (St), N-Methyloacrylamide and Ammonium per sulphate (APS) were purchased from Sigma Aldrich Chemicals Co. C.I. Pigment Red 2 was purchased from Shandong Chemical limited company (China). The self-made deionized (DI) water was used in the experiment.
Synthesis of PE-WPUA Hybrid Emulsion
The preparation process of PE-WPUAs was divided into three steps including synthesis of the pre polymer, neutralization, and dispersion. The pre polymer was prepared in a 500-mL round bottom, three-necked flask with a mechanical stirrer, thermo meter, and condenser. The pre polymerization of polyurethane pre polymer was based on IPDI, polyol, DMPA, BDO, DBTDL with acetone as solvent. The reaction was carried out at 80oC in a water bath under a nitrogen atmosphere for 2 h until the theoretical value of NCO groups was reached. The measurement of NCO content was carried out according to the standard titration method (ASTMD 2572-87).Then, HEMA was added sequentially into the reactor and reacted for 1.5 h. The double bond-end capped polyurethane pre polymer was obtained. After the pre polymer temperature dropped to room temperature, the pre polymer was neutralized by TEA for 15 min and the degree of neutralization was 100%. Finally, the vessel was cooled to room temperature, followed by dropping water with high speed stirring for about 0.30 h to obtain the PU dispersion with solid content of>40 wt.%.
The obtained above PU dispersion was introduced into a three- necked vessel, dispersed with water followed by adding emulsifiers AE09 and K-12, stirred at high speed and emulsified at room temperature for 10 min under a nitrogencondition. Then gradually added all the monomers required amount at 5 minutes interval and run about 30 minutes with continuous stirring and then initiated the polymerization temperature of 80°C for 4 h to obtain the hybrid emulsion according to our previous report [16]. Then, 1/6th part of emulsion was added in a three neck bottle flask and required water. After that, raised the temperature up to 75oC and simultaneously dissolve APS in 10 ml of distilled water and after the temperature reached to 75oC, 1/3thamount of APS added drop by drop until its color turned to blue. After observing blue beam remaining 5/6 parts of emulsion and 2/3 parts of initiators added into the reactor simultaneously one by one in 1 hour of intervals at 80oC. Again, increased the temperature ranges from (80-85oC) and continue to 30 minutes with low stirring speed. Afterwards, run the process till for getting sweet smell (If sweet smell was not obtained or strong smell observed then 10wt % extra initiator added into the mixtures, needed to run another 20 minutes at 85oC). After observing sweet smell, reduce the temperature at 40oC. Neutralization process was terminatedwith ammonia water (20 wt %) to the solutions mixtures until pH 7 was not achieved. By reducing the temperature of the solution and collected the entire residue through filtration media. Then washed the residue and dried it in oven at 105oC for 90 minutes. The polyester based waterborne polyurethane-acrylate (PE-WPUA) dispersion was thus obtained successfully. The hybrid emulsion samples are traced as PE-WPUA, PE-WPUA-3, PE-WPUA-5, PE-WPUA-10, PE-WPUA-15 and PE-WPUA-20 where the number represents the weight percentage of PU in the emulsion. The compositions of polyester based WPUA hybrid emulsion prepared is listed in Table 1.
Table 1: Chemical compositions of the synthesized PE-WPUA
| Samples |
PU composition (mol) |
Acrylic monomer (wt %) |
||||||||
| IPDI | Polyol | DMPA | BDO | HEMA | BA | AA | NMA | ST | MMA | |
| PE-WPUA | 1.4 | 0.25 | 0.65 | 0.14 | 0.72 | 66.10 |
2.10 |
1.60 | 8.40 | 21.80 |
| PE-WPUA-3 | 1.4 | 0.28 | 0.65 | 0.11 | 0.71 | 66.10 |
2.10 |
1.60 | 8.40 | 21.80 |
| PE-WPUA-5 | 1.4 | 0.3 | 0.65 | 0.12 | 0.76 | 66.10 |
2.10 |
1.60 | 8.40 | 21.80 |
| PE-WPUA-10 | 1.4 | 0.32 | 0.65 | 0.16 | 0.78 | 66.10 |
2.10 |
1.60 | 8.40 | 21.80 |
| PE-WPUA-15 | 1.4 | 0.26 | 0.65 | 0.13 | 0.73 | 66.10 |
2.10 |
1.60 | 8.40 | 21.80 |
| PE-WPUA-20 | 1.4 | 0.24 | 0.65 | 0.17 | 0.74 | 66.10 |
2.10 |
1.60 | 8.40 | 21.80 |
Pigment Printing
The print paste was prepared in soft water with heavy string by adding 4 g aforementioned synthesized WPUA emulsion separately, 0.4 g Red pigment, 0.8 g thickener, 6 g ammonia to attain a pH 8 – 9 and 0.7% – 0.8% w/w fixative were also added. The viscosity of the final print paste was determined with Brookfield viscometer. The homogenized printing pastes have been carried out to pure cotton fabrics using a flat screen technique. The samples printed were dried at 80oC for 3 min and thermo fixed at a temperature of 150oC for a period of 2.5 min.
Measurement and Characterization
The solid content of emulsion can be calculated by:
![]()
Where X denoted is the solid content of WPU or WPUA emulsion, W1 is the mass of WPU or WPUA and vessel before being put into the oven, and W2 is the mass of WPU or WPUA and vessel after being put into the oven. We have carried out the emulsion stability test for individual sample, color strength (K/S) and fastness values according to our previous reports[17, 18]. The viscosities of emulsions were measured on a Brookfield DV-II viscometer using UL adapter spindle. The surface tensions of emulsions were determined on OCA-20-LHT surface tensiometer (Dataphysics, Germany). The average particle size was measured using a Laser diffraction particle size analyzer (LS 13320, Beckman coulter, USA). The mechanical properties of the samples were measured at 25oC on an AGS-J Table-top Type Universal Tester (Shimadzu, Japan). The water absorption (ɷ) ratio was calculated as follows:
![]()
Where S1 is the weight of the raw dry sample, and S2 is the weight of the swollen sample. Measurement of yellowness resistance was carried out by using a spectrophotometer with 10o observer under the D65 light source. Tear strength of these coated fabrics were evaluated according standard test method, BS EN ISO 13937-2. The standard ASTM D 1269 test method was used to determine the crease recovery angle (CRA) using the Shirley Crease Recovery tester.
The functional groups of samples were measured by using a Fourier transform infrared spectroscopy (FTIR,Bruker, United Kingdom). The thermal degradation behaviour was determined with a thermogravimetric analyzer (TGA-Q500, TA Instruments, New Castle, DE).
Results and Discussion
Physical and Mechanical Properties of Prepared Emulsion
The physical and mechanical properties of prepared emulsions were recorded in Table 2.
Table 2: Physical and mechanical properties of PE-WPUA
| Sample code | Solid content(Wt %) | Viscosity(cP) | Avg. particle size (nm) | Surface tension (mN/m) | Tensile strength(MPa) | Elongation(%) |
| PE-WPU | 46.56 | 63 | 92 | 4.56 | 0.71 | 64.76 |
| PE-WPUA-3 | 44.78 | 71 | 85 | 4.62 | 0.78 | 55.89 |
| PE-WPUA-5 | 43.56 | 85 | 73 | 4.67 | 0.87 | 51.67 |
| PE-WPUA-10 | 42.89 | 87 | 68 | 4.72 | 1.10 | 45.80 |
| PE-WPUA-15 | 42.12 | 90 | 51 | 4.78 | 1.23 | 41.07 |
| PE-WPUA-20 | 41.35 | 93 | 43 | 4.85 | 1.56 | 37.80 |
These emulsion properties are considerable parameters play a vital roleto establish the economic cost and sustainable use of products. These parameters influence the processability of coatings, as well as the properties of cured films. One of the principle purposes of this research was to augment the solid content (%) of emulsion. This goal became performed efficaciously as amount of emulsions solid content was >40% for all samples as presented in Table 2. It was shown that solid content of all prepared samples are decreasing gradually and in this sense the results are similar of author’s thought due to the significance of lower solid content of emulsion in the surface layers statistically will enhance physical and mechanical properties as revealed by [19]. The indication of decreasing solid content of emulsion is increasing water content in the emulsion and it is used in surface layers to increases moisture content of surface particles and mat moisture of surface layers. Heat transfer into the core layer of particle emulsion increases during hot pressing due to high moisture content of surface layers. Surface layer particles are plasticized through water vapour. This plasticized surface causes tighter and greater compact structure within the layers. Thus, mechanical properties increases and water diffusion into the particle emulsion is troublesome [20]. Viscosity is an important feature of dispersions, which influence the processability and properties of coatings. It is acceptable that the viscosity of emulsion arises from the interplay between the particle-particle interactions (repulsive force between the similar charges surrounding the particles or H-H interaction between the hydroxylfunctional particles) and hydrodynamic interaction [21]. It’sshownfrom Table 2 that the apparent viscosities values of all the WPUA emulsions are more prominent than that of the pure WPU with the expansion of polyurethane content. This was attributed due to the increasing of carboxyl content in the molecular structure of waterborne polyurethane. Therefore, the amount of ions and counter-ions in the polyurethane molecule chain was increased both. At the same time, the strength of electric stagnate effect and ion hydration resulted in the thickness of the hydrated membrane. Finally, the effective volume of ions was showed greater than the true volume of the original particle. Furthermore, the hydrated film thickness and the particle hydrodynamic volume accelerated resulted in the emulsion viscosity increased. The average particle sizes of synthesised WPUAs were determined by using a Laser diffraction particle size analyzer. As shown in Table 2, the average particle sizes of final products were decreased within the PU concentration due to the number of particles increased, the average distance between the ions decreased, that means the chance of any two particles entered into the mutual attraction area then displacement became not easy and the particle sizes decreased. This small difference in sizes can be correlated with soft segment chemical structure. The polyester polyol contains more polar structure due to polar carbonyl groups in ester linkages. The particle size of final compound depends upon several factors such as hydrophilicity of monomers, molecular weight of pre polymer and flexibility of fundamental chain. It is essential to mention that the particle size has an immediate impact on the emulsions stability which determines their secure storage duration. The emulsions with larger particle sizes (>1000 nm) are generally unstable, while with the smaller particle sizes (<200 nm) are considered to be storage stable [22]. However, the control of the mean particle size is important with respect to the specific utility of WPUA dispersion. For example, relatively larger particles are preferred in surface coatings for rapid drying, and smaller ones are desirable when the deep penetration of the dispersion into a substrate is essential. In any issue, it was found that there was no problem with using WPUA emulsions prepared here as adhesives for textile application. The wettability of emulsions on the substrates is considered important for adhesion properties, while the surface tension is an imperative physical property parameter for the application performance of the emulsions. The wettability of WPUA emulsions were measured by the surface tension. As seen in Table 2, the surface tension of the WPUA emulsions was increased slightly with the increased of PU amount. This may be happened due to the migration of ester linkage units to the WPUA surface layer and also the electronic cloud density around the PU chains were considered higher than that around the acrylate monomer, particularly in soft segments [23]. It is known that, the surface tension is influenced by the surface state i.e., component and structure and the environment temperature. Although the temperature coefficient of a polymer surface tension is not very large, the effect of temperature on the surface property is crucial in some conditions [24]. Mechanical properties of the emulsion are of important tone that must be taken into consideration when the emulsion is utilized as binder for the pigment printing due to the color fastness and handling of the printing goods are emphatically accelerated by the mechanical properties of the binder film, particularly the elongation at break and the tensile strength [25]. Table 2 shows the tensile strength increases continuously from 0.71 MPa to 1.56 MPa with increasing of PU amount from 3 to 20 ml, whereas the values of breaking elongation show the opposite tendency. These mean that the addition of PU complements the mechanical properties of the emulsion. The largest tensile strength may be due to the H-H bonding density and better phase separation and PA was harder than PU, which increased the rigidity and strength and reduced the toughness of the hybrid emulsion films. In PU chain HEMA may react with the acrylate monomers that help to form three-dimensional network structure between the two chains. To some extent, network structure prevents molecules from sliding, which ends within the increment of stress and strength [26].
Water Absorption and Yellow Resistance
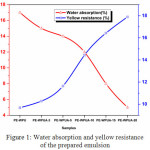 |
Figure 1: Water absorption and yellow resistance of the prepared emulsion Click here to View figure |
For WPUA used as adhesives, the clearest deformity is poor water resistance property. In this concern, numerous researchers have been focused on the improvement of water resistance property of WPUA [27]. Mainly, water resistance characteristics of polymers are dependent on their chemical and physical structure. Hydroxyl (-OH) and carboxylic acid groups of PUA that is responsible for increasingH-H bonding interaction of emulsion and they are sensitive to water. Taking notice incase of lower surface tension of WPUA emulsion, polyurethane addition can be an option to enhance the water resistance of WPUA. Figure 1 shows the water resistance of prepared WPUA samples. The results revealed that the water-resistance of WPU was very poor. It is clearly demonstrates that the absorbed water ratio decreases with the increase of the PU content. PU has an obvious influence on the absorbed water ratio. With PU content increases from 3 to 20 ml, the absorbed water ratio decreases from 17.23% to 4.08%, the water absorbability administers obvious decrease with little PU content. The covalently crosslinking density is superior in case of increased HMEA/BA weight ratio. Because of this, water is not able to enter easily into the cross linked macromolecules and their water resistance is advanced [28] and another reason may be due to the fact of molecular-weight polyester polyol was conducive to the formation of a compact layer during the curing course of emulsion, so water rarely permeated this impediment to the inside. It can be decided that by introducing hydrophobic polyester polyol into soft segment of polyurethane chains, the water-resistance of WPUA material can be more desirable without problems. From the Figure 1, yellow resistance improved gradually at a regular rate. This could be due to that, at the first PUA sample emulsion the percentage of polyurethane was less compared with last sample emulsion.
Stability Performance
Table 3: Stability performance of PE-WPUA hybrid emulsions
|
Samples |
Electrolyte stability | Dilution Stability | Acid and alkali stability | Storage stability |
| PE-WPU | Good | Pass | Stable | stable |
| PE-WPUA-3 | Average | Pass | Stable | stable |
| PE-WPUA-5 | Satisfactory | Pass | Stable | stable |
| PE-WPUA-10 | Good | Pass | Stable | stable |
| PE-WPUA-15 | Good | Pass | Stable | light stable |
| PE-WPUA-20 | Good | Pass | Stable |
unstable |
Table 3 represents the stability test performance of prepared binder samples. Electrolyte stability is considered to be the most important parameter of the emulsion stability. To our best knowledge, the electrolyte stability of the latexes was influenced by electrostatic force, Van der Waals’ force, solvation, steric stabilization effect, and surface tension. In electrolyte resistance stability test it can be observed that samples (PE-WPU, PE-WPU-10, PE-WPU-15 and PE-WPU-20) were showed good stability and other samples (PE-WPU-3 and PE-WPU-5) are performed normal to agreeable performance ranges without any precipitation. The cause is probably with increase in electrolyte, the concentration of electrolyte in the water would increase, provides the thin electric double layer and reduction of zeta potentials. The less magnitude of the zeta potential resulted in the latex system with worse stability against coagulation [29]. This result counseled that the emulsion polymerization supported WPUA hybrid latex may exhibit more advantageous storage stability. The dilution stability of all prepared samples has been passed by lab scale experiment. Furthermore, acid & alkali stability test also showed stable phenomena for all samples. A stoppered glass bottle, containing 100 mL of the WPUA emulsion (with different ratios of PU) was stored for three month to check the storage stability. After every period, it is discovered that a few emulsions have settled down into two layers. It is understandable that with increased PU amount in the emulsion delivered a rapid separation of the emulsion into two layers and formation of a paste which is found more stable than others but after 10 ml stability was reached to nil i.e. unstable. According tothe emulsion polymerization theory, the diameters of latex particles trend to raise to keep the emulsion stable [30]. It might be related another issue that extra amount of sodium dodecyl sulfate used leads to the viscosity increases slightly and the storage stability decreases sharply becausesodium dodecyl sulfate is a kind of organic salt and the usage of excess sodium dodecyl sulfate in dispersion produces salt-out effect [31]
FT-IR Analysis
The chemical structures of the WPUAs have been investigated by FT-IR spectroscopy technique. As shown in Figure 2, similar bands compared to WPU sample without significant shift in peaks position, and furthermore, all spectra confirm the formation of well-defined polyurethane-acrylate structures. The characteristic peaks at 3264 and 1720 cm−1 were assigned to –NH and –C=O stretching vibration, respectively. It can be seen from Figure 2(b) that, there is a dynamic change in the absorption pattern of C=O stretching region, which may be attributed to the presence of acrylate group [32].The bands at 1722 cm−1 (amide I, υC=O), 1531–1542 cm−1 (amide II, δN–H and υC–N), 12442 cm−1 (amide III, υC–N and δN–H) and 1138–1154 cm−1 (antisymmetric υC–O–C) confirm the formation of urethane group and successful grafting of acrylate monomers onto reactive site of polyurethane emulsion [33]. Moreover, the absence of a band at 2274 cm−1 confirms that unreacted NCO groups are not present, which is a result of the fact that when NCO reacted with active hydrogen of a hydroxyl functional compound (hydroxylated polyester polyol), urethane linkage was formed [34]. Since we are keen on confirmation of occurrence of chemical grafting of acrylates onto polyester backbone of polyurethane dispersion, it is necessary to check the absorption region of C=C bond. The band at 1640 cm−1 in Figure 2(d) indicates the incorporation of C=C bond in its macromolecular chain, however, the absence of this band in hybrid structure of PE/WPU-10, is the evidence of grafting during copolymerization. Furthermore, both C=O and N-H peaks are relatively broader for PE/WPU-10 indicating more enhanced phase mixing in the polyester-based soft segment. The samples Figure 2(c), (e) and (f) exhibited similar absorption pattern as observed in case of rest of the hybrid emulsions, hence need no discussion. These outcomes are in concurrence with perceptions recorded by thermal analysis of latex.
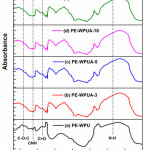 |
Figure 2: FTIR spectra of different WPUA emulsion samples Click here to View figure |
TGA Analysis
Thermal stability of polymers is generally determined by using TGA analysis. TGA curves identified the weight loss (%) of materials with respect to the temperature of thermal degradation. The thermal stability of all samples was analyzed in the range of 0–700oC and the outcome is appeared in Figure 3. Thermal degradation of WPUA is a very complex mechanism due to variety of products formed in the process which can be indicative of the complexity of the degradation manner. Thermal stability of WPUA emulsions mainly depends upon the number of hard and soft segments present in the final structure. The urethane linkages (–NH–COO) and structure of isocyanate used for curing are responsible for the hard segment while long alkyl chains of polyols presents soft segments in the final cured structure. The decay stages of the emulsion was separated into three phases, primary stage weight reduction happens at around 200–245oC because of the relatively low thermal stability and the second stage, most of the hard and soft segments of PU were decomposed, and the weight loss reached a maximum that headed to 77–85% in the range 300–352oC. Finally, a small numbers of carbon chains and residual impurities were decomposed to a minimum when the temperature was 550oC. All samples had great thermal stability in the first stage, but PE-WPUA-10 exhibited more perfect thermal stability than others duringthe temperature over 270oC due to molecular weight that raised the chemical bond and entanglement among the molecular chains. Tao Wu showed that oligomer polyol molecular weight turned into conducive and resultant improvement of emulsion thermal stability [35].
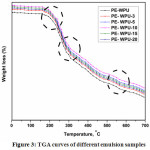 |
Figure 3: TGA curves of different emulsion samples Click here to View figure |
Color Strength and Fixation Properties
It is well-known that binding agent received a great interest for fiber affinity because pigments have no affinity on the fiber. The binder thermally cured on the fabric and produces a continuous film that fixes the pigments to the fabric. Table 4 shows the colour strength (K/S), color fixation i.e., fastness properties and hand feeling of cotton fabrics that printed with commercial binder and synthesized WPUA hybrid latex separately. The commercial binder providessmall color strength values as compared to synthesized hybrid latex at the same conditions, indicating that the uniformity of pigment printing with synthetic latex was superior to that with commercial binder. The rubbing fastness for printed samples using prepared binder is an advanced as compared to commercial binder. As the binder concentration extended, the rubbing fastnesses have been decreased even as the washing fastnesses have no obvious exchange. This is because more binder particles adsorbed by cotton fiber surfaces, which determined the rubbing fastness decreased. The rubbing fastness depends on binder elasticity, adhesion on the textile materials, and uneven dispersing of the pigment in the print paste [36]. WPUA emulsion plays an ideal role in the improvement of washing fastness which may be attributed to the formation of insoluble lakes caused by electrostatic interaction and the recoverable isocyanate groups and stronger covalent bonds generated between cotton fabric and emulsion. Overall, the participation of H-H bonds between hydroxyl groups on the surface of prepared binder and the hydroxyl groups on the surface of the cotton fiber in the adhesion process could enhance the pigment adhesion to the fiber. It was also shown that all of the samples printed using the synthesized binder acquires a soft handle but after 10 ml PU became hard feel. The particle size is the main reason for improving the performance of binder latex [37], and the soft polymer composition wasselected to suit the textile printing, which requires the soft handle for printed products. This was true irrespective of nature of the binder used or the type of fabric printed.
Table 4: Color strength, overall fastness and handle properties of printed cotton
| Samples | K/S | Rubbing fastness | Washing fastness | Handle | ||
| dry | wet | Alt | St. | |||
| commercial binder | 8.43 | 3 | 1-2 | 3 | 3 | hard |
| PE-WPUA | 9.11 | 3-4 | 3-4 | 4 | 4 | soft |
| PE-WPUA-3 | 9.26 | 4 | 4 | 4 | 4 | soft |
| PE-WPUA-5 | 9.64 | 4-5 | 4-5 | 4-5 | 4-5 | soft |
| PE-WPUA-10 | 10.62 | 4-5 | 4-5 | 4-5 | 4-5 | soft |
| PE-WPUA-15 | 10.42 | 3-4 | 3 | 4-5 | 4-5 | hard |
| PE-WPUA-20 | 9.82 | 3-4 | 2-3 | 4 | 4 | hard |
Physical Properties of Treated Fabric
Tear strength is one of the regular fabric utility parameter that plays a role to make decision their quality and end uses. In this study, cotton fabrics were treated by using synthesized WPUA emulsions and resultant tear strength betterment is showed in Table 5. It is generally accepted that, cotton is composed of 99% cellulose in nature and anhydro-β cellobiose is the repeating unit in cellulose long chains. Three hydroxyl groups (–OH), one primary and two secondary, in each repeating cellobiose unit of cellulose are chemically reactive. During polymerization, hydroxyl groups may interact with polar –NH and C=O groups of WPUA emulsions through polar interactions and H-H bonding resulting in prominent fabric tear strength. Improvement in tear strength values of polyester-based emulsions was observed in following order: untreated < PE-WPUA < PE-WPUA20 < PE-WPUA-3 < PE-WPUA-5 < PE-WPUA-15< PE-WPUA-10.
Table 5 represents the crease recovery angle data of cotton fabric before and after treated by WPUA emulsion. It was shown that, with the increase in PU concentration, crease recovery angle was increased. Cotton fibres consists of large number of hydroxyl groups causes creasing problems due to the snarling of cellulose macromolecules during twisted, rubbed, washed or worn. WPUA emulsion plays a crucial role to reduce the snarling by forming network between the fibres and eventually causing to improve the crease recovery of the treated fabric. The result might be appeared due to the following reasons: Firstly, polyester based emulsion contain unique structure and small particles so that it is able to penetrate easily into microstructure of the fibre and formed continuous thin film. Secondly, polyester polyol was used as adjuvant of emulsion network structure with fibre due to the presence of its active groups [38], and the formed network limited the movement of macromolecular chains in the amorphous field in the fibre. Besides, it can be seen that increment rate of crease recovery angle of treated fabric became low when the concentration of PU surpassed 10 ml.
Table 5: Physical properties of treated samples
| Samples | Tear strength (N) | CRA (deg) |
| commercial binder | 25 | 35 |
| PE-WPUA | 38 | 42 |
| PE-WPUA-3 | 48 | 51 |
| PE-WPUA-5 | 67 | 58 |
| PE-WPUA-10 | 82 | 88 |
| PE-WPUA-15 | 52 | 73 |
| PE-WPUA-20 | 30 | 63 |
Conclusion
A series of novel PE-3030 based waterborne polyurethane/acrylate (WPUA) hybrid emulsions were successfully prepared by emulsion polymerization with acrylate monomers. FT-IR spectra analysis ascertains the successful formation of WPUA. TGA showed that the thermal stability of the WPUA emulsions was enhanced by increasing the PU content. With the increment of PU, the viscosity and surface tension both were increased. With PU content increases from 3 to 20 ml, the absorbed water ratio decreases from 17.23% to 4.08%. The tensile strength was changed from 0.71 MPa to 1.56 MPa after the PU was treated with PA emulsions and lower breaking elongation. Comparison with a certain commercial binder suggests that the PE-3030 based WPUA latex has a charming expectation on industrial uses.
Acknowledgement
The authors wish to acknowledge the Runhe chemical industry, china & Color root (Hubei) technology limited, china, for providing the technical support and the school of chemistry & chemical engineering, Wuhan Textile University, china, for providing chemicals and all measurements.
References
- Madbouly, S. A. and Otaigbe, J. U.,Pro. in Poly. Sci., 2009. 34,1283-1332
- Hirose, M., Kadowaki, F., and Zhou, J.,Pro. in Org. Coat.,1997. 31,157-169
CrossRef - Chai, S. L. and Tan, H. M.,J. of app. poly. sci., 2008. 107,3499-3504
- Potolinca, V. O., Buruiana, E., and Oprea, S., J.of Poly. Res., 2013. 20,237
- Oprea, S.,J. of app. poly. sci., 2007. 105,2509-2515
- Nohra, B., Candy, L., Blanco, J.-F., Guerin, C., Raoul, Y., and Mouloungui, Z., Macromolecules, 2013. 46,3771-3792
CrossRef - Oertel, G., ed. 1993, Hanser Publishers: New York.
- Hao, H., Hu, J., Wang, F., and Tu, W. in IOP Conference Series: Mat. Sci. and Eng.. 2017. IOP Publishing. 012067
- Fan, W., Du, W., Li, Z., Dan, N., and Huang, J.,Pro. in Org. Coat.,2015. 86,125-133
- Wang, L., Shen, Y., Lai, X., Li, Z., and Liu, M., J. of Poly. Res., 2011. 18,469-476
CrossRef - Chattopadhyay, D. K. and Raju, K.,Pro. in Poly. Sci., 2007. 32,352-418
- Peng, S., Jin, Y., Sun, T., Qi, R., Fan, B., and Cheng, X.,J. of app. poly. sci.,2014. 131,40420
- Lee, T., Kwon, S., and Kim, B.,Pro. in Org. Coat.,2014. 77,1111-1116
- Šebenik, U., Golob, J., and Krajnc, M., Poly. int., 2003. 52,740-748
- Howarth, G., S. coat. int. part B: coat. trans., 2003. 86,111-118
- Sahil, M., Khan, M. M. R., Pervez, M. N., Anshu, C., Habib, M. A., and Quan, H., A. j. of chem., 2017. 29,1145-1149
- Mahmud, S., Habib, M. A., Pervez, M. N., and Islam, A., A. J. of Poly. Sci. & Eng., 2015. 3,119-128
- Pervez, M. N., Inamdar, U. Y., Talukder, M. E., Mahmud, S., Habib, M. A., Kamruzzaman, M., and Cai, Y., MATEC W. of Con., 2017. 108,03002
CrossRef - Sarı, B., Nemli, G., Baharoğlu, M., Bardak, S., and Zekoviç, E., J. of Com. Mat., 2013. 47,1247-1255
CrossRef - DeXin, Y. and Östman, B. A.-L., Eur. J.of W. and W. Pro., 1983. 41,281-286
- Guo, Y.-h., Li, S.-c., Wang, G.-s., Ma, W., and Huang, Z.,Pro. in Org. Coat.,2012. 74,248-256
CrossRef - Kim, B. K. and Lee, J. C.,J. of app. poly. sci., 1995. 58,1117-1124
- Asif, A., Huang, C., and Shi, W.,Coll. and Poly. Sci., 2004. 283,200-208
CrossRef - Feng, L., Zhang, X., Dai, J., Ge, Z., Chao, J., and Bai, C.,Fro. of Chem. in Ch.,2008. 3,1-5
CrossRef - Zhang, J., Li, X., Shi, X., Hua, M., Zhou, X., and Wang, X.,Prog. in Nat. Sci.: Mat. Int.,2012. 22,71-78
- Li, X., Fei, G., and Wang, H.,J. of app. poly. sci., 2006. 100,40-46
- Pan, H. and Chen, D.,Poly.-Pla. Tech. and Eng., 2008. 47,595-599
CrossRef - Ma, G., Guan, T., Hou, C., Wu, J., Wang, G., Ji, X., and Wang, B., J. of Coat. Tech. and Res., 2015. 12,505-512
CrossRef - Yu, Y., Wang, J., and Zong, J.,Des. Mono. and Poly.,2015. 18,242-250
CrossRef - Smith, W. V. and Ewart, R. H.,The j. of chem. phy.,1948. 16,592-599
- Hou, Z.-s., Qu, W.-q., and Kan, C.-y., J. of Poly. Res., 2015. 22,1
CrossRef - Athawale, V. D. and Kulkarni, M. A.,Pro. in Org. Coat.,2009. 65,392-400
CrossRef - Zhang, H., Huang, H., and Cao, J.,J. of app. poly. sci., 2004. 94,1-8
- Wang, X., Hu, Y., Song, L., Xing, W., Lu, H., Lv, P., and Jie, G.,S. and Coat. Tech., 2010. 205,1864-1869
CrossRef - Wu, T., Xin, X., Liu, H., Xu, B., and Yu, X.,J. of app. poly. sci., 2016. 133,
- Hamilton, L. E. and Chiweshe, A.,Sta.‐Stär.,1998. 50,213-218
- Abdou, L., El-Molla, M., Hakeim, O., El-Gammal, M., and Shamey, R.,In. & Eng. Chem. Res., 2013. 52,2195-2200
CrossRef - Wang, H., Huang, C., and Liu, N.,Adv. Mat. Res., 2011. 331,382-385

This work is licensed under a Creative Commons Attribution 4.0 International License.









