A Nano Catalyst of Cofe2o4@ B18N18 as A Novel Material
Matin S. Moosavi, Majid Monajjemi and Karim. Zare
Department of Chemistry, Science and Research Branch, Islamic Azad University, Tehran, Iran.
Corresponding Author E-mail: m_monajjemi@srbiau.ac.ir
DOI : http://dx.doi.org/10.13005/ojc/330408
In this work the properties of CoFe2O4@ B18N18have been illustrated as a novel catalyst to compare with well-known catalyst “Fe3O4@Silica”.It has been shown that CoFe2O4 magnetite particle can be use as important catalyst inside the B18N18 ring. In our previous papers amazing result about the BnNn properties have been calculated, exhibited (Struct.Chem., 23, 551-580, (2012); J. Phys. Chem A, 117, 1670-1684, (2013); J. Phys. Chem. C, 114, 15315,(2010)and discussed. In present work it has been shown there is a non-covalent attraction between CoFe2O4 and B18N18 coated molecules. In the system of CoFe2O4@B18N18 catalyst, the magnetic nanoparticles (MNPs)as a core part causes important change in the electronic structure of B18N18 as a shell which some compounds are specific-sensitive functional groups for this system such as OH, CHO,NH2 and were subjected to the some organic reaction.The Physical-chemistry properties such asenergy densities, potential energy densities, electron densities, ELF, LOL,eta index, ellipticity of electron density and ECPforCoFe2O4@ B18N18 shell have been calculated and simulated in related reactions for those groups-functionalized. Our Calculation indicates that the B18N18 is much better surfaces for CoFe2O4 rather than silica surfaces.
KEYWORDS:CoFe2O4; Nano-Particles; electron density; B18N18nano-ring; Silica; SiO2
Download this article as:| Copy the following to cite this article: Moosavi M. S, Monajjemi M, Zare K. A Nano Catalyst of Cofe2o4@ B18N18 as A Novel Material. Orient J Chem 2017;33(4). |
| Copy the following to cite this URL: Moosavi M. S, Monajjemi M, Zare K. A Nano Catalyst of Cofe2o4@ B18N18 as A Novel Material. Orient J Chem 2017;33(4). Available from: http://www.orientjchem.org/?p=35192 |
Introduction
CoFe2O4 as the Cobalt ferrite crystallizes in a partially inverse spinel position represented as (Co2x++Fe3+ 1-x)(Co2+ 1-xFe3+ 1+x)O4 where x based on thermal1 condition . It is ferri-magnetic with a Tc approximately 520 degree and exhibits a relatively magnetic hysteresis which distinguishes it from the other of the spinel ferrites2, 3. Magnetic measurements on nano-particles of cobalt ferrite dispersed in various solvents of organic compounds and nano-crystalline2 powders prepared by hydroxide precipitation3-5 have been investigated earlier. In magnetic fluids3, it has been seen that for particles above threenanometer the saturation3,4magnetization remains constant at about 30 emu g-1 that is extensively less than the bulk5 values.
Magnetic nanoparticles (MNPs) have shown exceptional potential for several biological and clinical applications4, 5. However, MNPs might be coated by the biocompatible shells for such applications. The aim of this study is to understand if and how the surfaces charges and coatings can affect the magnetic and electronic properties of Cobalt ferrite crystallizes. The role of the surfaces on the magnetic moments of a magnetic nano particle such as CoFe2O4 is an important issue, and various effects can contribute for making it deviate from the bulk value, including the charges, the nature of the coating, and also the synthetic technique. The electronic properties and ionic distribution of CoFe2O4 NPs were probed by X-ray2-4 absorption spectroscopies X-ray-magnetic-circular-dichroism and X-ray-photoemission-spectroscopy-techniques known as the abbreviation XAC, XMCD and XPS respectively. Magnetite-particles1-5 is also of interests in medicinal and industries application such as magnetic-resonance-imagingor {MRI}, organic catalyst and nanomaterial synthesize6-8.
The overall magnetic behavior and the hyper-thermic properties were evaluated by magnetometers and molecular modeling measurements, respectively7,8. The results show that all of the investigated CoFe2O4 NPs have high magnetic anisotropy6,9energies, and the surfaces charges and coating do not influence appreciably their electronic and magnetic properties. In addition, the citrate shell improves the stability of the NPs in aqueous environment, making CoFe2O4 NPs suitable for biomedical applications.Magnetic nanoparticle exhibitsseveral unique properties such as super-para-magnetism compared to bulk9,6material andparticularly, are used in the field of biology7-9 and medicines. Magnetic nanoparticle has attracted a great deal of researchinterests due to their distinctive properties and special applicationrecently.
CoFe2O4, is a well-known hard magnetic material with very high cubic magneto-crystalline anisotropy, high coercively, and moderate saturation magnetization. These properties make it a promising material for high-density magnetic recording.
CoFe2O4 nanoparticles have been widelysynthesized using severalways, same as sol-gel9,10, micro-emulsion11,12, chemical coprecipitation11,12, hydrothermal synthesisand microwave11-13 synthesis14. Among those methods, thesol-gel9,10routesare very attractive, within the main advantages includingsimple control for chemical composition11. Recently, in thesol-gel9,10synthesis of CoFe2O4 particles, the gelsare built-up via physical and chemical binds between the chemical species11-14. It has been introduced a different sol-gel9,10routes@ polyacrylamide gel route for preparing CoFe2O4 nanoparticle.Due to lack of controls over the specific transformation of ananoparticle, obviously super-paramagnetic particle has not been prepared from magnetite, i.e. Magnetite-nano-particle whichgenerally loses their permanent magnetic properties in the lake ofthe external magnetic field6-14.
The most applications require at least a magnetic-particle for dispersing in the non-magnetic matrixes. Thismatrix plays an important role for providing the meaning of particle dispersion for determining a physical property of a composite10,14
The other important items of these matrixes are to act as a protection for magnetic nano-particlesagainst oxidation or corrosion especially13,14in the metallic nano-particles15-17. Among oxide matrixes15 such as alumina,silica16, zeolites,titanic oxides, carbon-based, the silica16 can be a general suitable materials for the matrixes because of inertness ofthe magnetic fields, its non-toxicity and easiness for forming crosslined networks structure14-17.Silica shells chemically is stable and can be rapidly functionalized in the bio-conjugation purposes, in other words is biocompatible therefore CoFe2O4@SiO2 as a silicacoated magnetite composite nanoparticles have been synthesized by several groups18-20.
Recently, silica coated magnetite functionalized by γ mercaptopropyl-tri-methoxysilane has been successfully used for extracting Pb2+, Cd2+,Na+, K+, Mg2+, Hg2+, and Cu2+in the wide pH ranges from water15-20. The complex of metal-CoFe2O4@SiO2–NH2 nanoparticles1-4 could be recovered easily from aqueous through magneticseparation and reproducereadily by acid treatment. By this work it has been exhibited the amino-functionalized CoFe2O4@B18N18 magnetic nanoparticles compare to CoFe2O4@SiO2is much more effective as recyclable adsorbent for the removal of heavy and alkali/earth metal ions in water and wastewater treatments.
Catalysts has a very sensitivetreatment in technology and modern sciences as they increase reaction yield via reducingthe temperature in synthesisof the chemical product21,22.There are two basic types of catalysis, (1); “heterogeneous22-23, where the reaction accomplishes on the surfaces and the catalystsare in the solid phase.(2) “homogeneous23, where the catalyst is in one phase as reactant21.
The heterogeneous22 catalyst might be easily separated from the mixed solvent but because of their limited area of surfacesthe reaction rate is restricted22. Meanwhile homogeneous23 catalyst might react fast for providing a good rate (conversion rate)for the catalyst, but as they are solvable in the medium reaction, it might be a laboring processingfor removing them of the reaction enviroment23. The problem in removing homogenous23, catalysts from the reaction environment leads to problem ofmaintain the catalyzer for repeating24. The bridge between heterogeneous22 and homogeneous23 catalysts can be attained through the CoFe2O4nanoparticle25.CoFe2O4exclusively is useful and important as the magnetic nano-particlewhich exhibit strong magnetic-momentand are seldomsustained outside of an external magnetic field25. These kinds of nano-particle might be consist of several materials such as nickel, cobalt, iron oxides, ferrites26,27and also alloys such as platinum/iron26.CoFe2O4 MNPs of silica shells catalytic materialshave the benefit for increasing surface area which causes for any increased reaction rate28. Moreover, nanoparticle might permit additional catalytic functionalitybecause of their unique properties29. Several catalysis of magnetic nano-structure has been investigated up to now30-32,such as preparation of nano-composite materials consist of magnetic-(core)nano-particle which has been coated by various shells of other catalytically30active nano-material32.
Other type of catalyststhatare interest for organic compounds involves the using of organic molecules which are enabling forpreservationthe materials in the end of any reactions for reuseing31-32. In this work we have investigated the catalysis’s properties of CoFe2O4 nanoparticles @B18N18 instead of SiO2 for comparing in the area of chemical synthesizes.
Recently extensive theoretical and experimental studies have been accomplished on boron-nitride-fullerenes for understanding their relative stabilities and also sizedependence of important physical properties33-35. (BN)n are iso-electronic with carbon species, making them the goal of various research area and enhanced34 by this fact whichB-Ncompounds havea stable crystallinephase near to graphite36-38. Electronic properties of differentBnNn rings and cages have been investigated theoretically39-45.Despite those experimental and theoretical works, the structural synthesized ofBnNn rings and cages are still unknown.
Boron nitrides exist in several crystalline forms, such as hexagonal and cubic shapes. Due to the closely structures between C-C and B-Nunits, large efforts have been donerecently to “BN” fullerenes, which has excellent properties like structural stability, heat resistance and insulation46,47 . As the thermodynamic mechanism ofBN growth (from nuclei) is still not wellknown for those nanotubes, a comprehensivetheoretical simulation continue to attractenhanced attention48. Although those nanotubes are found to be non-chiral or chiral;however, most interestsin the zigzag and armchair investigation are much less known about the chirality area of hexagonal boron nitride49.
In our previous works50-69, it has been exhibited the BN stabilities, NMR data, electronic properties, chemical phenomenon and the mechanism of tubes generatingfor the various structures of BN especially for the SWBNNT via a multi-walled nanotube including chirality m,n(with n = 3-4 and m=5-7). The diagram generation fornano-ring of the BnNn (n = 15, 16, 18 and 20) areshown in the references of52-54. In addition, we reported a unique stable structures52-54 with combinations of five and six loops same as a quasi B3N3H6 shape forB15N15 and B18N18, respectively. Those kinds of shapes have significant properties in the nano studies of CoFe2O4@ B18N18 particles. Thesuitable stability and reasonable aromaticity of those structures have been confirmed by thermodynamic data,frequency optimization and NQR52-54.
Hyperfine parameter and spin density, electrical potentials,electromagnetic properties and isotropic-fermi-coupling-constant52-55 indicate thestability of those rings through using non-bonded interactionmodel between CoFe2O4& B18N18.A novel aminofunctionalized CoFe2O4& B18N18 magnetic nano-material with the core-shell structureshas been developed, for removing heavy metal ions from the aqueous media.The elasticity of electron density, electrical properties same aselectron and energy densities, kinetic and potential energiesof densities, ELF or LOL,eta index and ECPforCoFe2O4-B18N18 core-shell systems have been calculated and simulated in related reactions for Amino-functionalized CoFe2O4@B18N18 core–shell magnetic.The amino-functionalized CoFe2O4& B18N18nano-adsorbent exhibited high adsorption affinities for aqueous Fe (iii), Pb (ii), Ni (ii), Cu (ii) and Zn (ii) ions, resulting from complexation by surface amino groups.
Background and Methodology
Magnetic particles are suitable for aqueous transition and heavy metals due to thier unique advantages of quick separation and their high surface area under external magnetic fields68-71. The surface modification, adsorption affinity, including covalent binding and physical coating, has often been explored70-75 for enabling specific complexationfor further facilitates72-76.
Recently it have been exhibited which the amino-functionalized molecules demonstrated outstanding abilities for removing a wide variety of transition heavy metal ions77-79. Both B18N18& SiO2are stable under acidic conditions, as compared with someothermaterials and functions for protecting the inner magnetite core80-82.
Although CoFe2O4@SiO2 has recently been investigated for potentialbiomedical applications82, there is no work about the CoFe2O4@ B18N18.
In this study with the theoretical approaches magneticnanoadsorbent has been developed via covalently grafting amino groupsover the surfaces of CoFe2O4@ B18N18nanoparticles. Part of the systems including CoFe2O4@ B18N18nanoparticles has been simulated with QM/MM methods and the investigation carried out by the Monte Carlo calculations.In this study, various force fields are done via “Amber” and OPLS for comparing the calculated energy of the CoFe2O4@ B18N18 nanoparticles. Furthermore, a Hyper-Chem professional release-7.01program is used for any further calculations.
For the non-covalent forces of B18N18andCoFe2O4, the B3LYP&BLYP methodsare unsuitablefor describing van der Waals83, 84 through medium-range interaction84. Therefore, the ONIOM* methods withthree levels of tight (H), medium Hamiltonian (M), andlow (L) calculations have been accomplished in these studiesto estimate the non-bonded interaction between B18N18and CoFe2O4.
Thedensity functional method is used for the high levelwhile the semi-empirical (pm6) with pseudo=Lanl2 and Pm3MM for both of them respectively. Some accurate studies have indicated that in-accuracy of the low range exchange energies goes tothe large systematic errors for the prediction of molecular properties85-88.
Geometries optimization and electronic calculations have been accomplished using the m06 functional of DFT. These approachesare based on solution of the Kohn & Sham equation89 in the plane-waves sets with projector-augmented-pseudo-potentials90. The Perdew&Burke&Ernzerhof PBE90exchange-correlation and generalized-gradient-approximation GGA90are also used for non-bonding calculation.
The charge transferring and electrostatics potentials derived charges were also estimatedusing the Merz&Kollman&Singh91, chelpG92or chelp93.The charges calculation methods based on MESP or molecular-electrostatics-potentials fitting are not wellsuited for the larger systems whereas several of the inner-most points are located far away from the centers at which the MESP93are computed.In that position, variation of the inner-most atomic charges would not be towards the changing of the MESP91 outside of the molecules.
The charge-density profiles of this study have been estimated from the first principle calculation through an averaging process as described in the references90-95. The interaction energies or adsorbents energies between metals and CoFe2O4@B18N18catalyst were done according to the equation as follows:
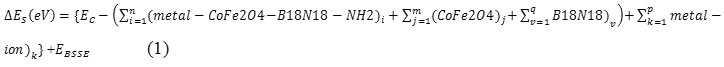
That ΔEs is the adsorbents energies.
Theelectron-localization-function or ELF96-98, localized-orbital-locator or LOL96-98 , electron density of theGradient-norm&Laplacian, values of orbitals wave-functions, electron spin densities, electrostatic-potentials from nuclear-atomic-charges,the exchange-correlation density, as well astotal electrostatic potentials ESP), correlation-holes and correlation-factors, and the average local ionization energies using the Multi-functional-Wave-function analyzer have also been calculated in this study96-98 .
Density Electron Approach for Interaction Between Mnps and B18N18:
The kinetic energies densitiesare not defined individually, since the expected values of the operators:
![]()
Can be estimated by integrating kinetic energy densities from those alternatives definitions. One of the usual used definitionsis as follows:
![]()
thelocal kinetic energies given below guarantee96-98 hence the physical dataare more commonly used. The Lagrangian of kinetic energies densities, G(r)97are also known as positive definite kinetic energy densities.
![]()
K(r) and G(r) are directly related by Laplacian of electron density
![]()
The electrostatic potential from nuclear/atomic charges can be calculated via:
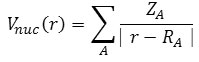
Where RA and ZA denote position vector and nuclear charge of atom A, respectively.
Becke and Edgecombe have noted that spherically averaged like-spin conditional pair probability have correlation with the Fermi hole and it has been suggested which the electron localization function (ELF)98.
![]()
Where
![]()
and
![]()
for close-shell system, since
![]()
D and D0 terms can be simplified as
![]()
In which the kinetic energies terms in D(r) is replaced by Kirzhnits types second-order gradients expansion, which are
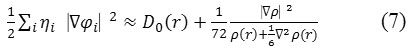
So that ELF is totally independent from the wave-function, and then can be used for analyzing electron densities from X-ray diffraction data.
Localized orbital locator or LOL98,99is another item for locating high localization regions likewise ELF, which explained by Schmider&Becke99 .
![]()
where
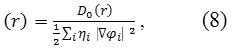
Do (r)
For spin-polarized system and close-shell system are defined in the same way as in ELF98. LOL have similar approaches compared to ELF.
Notice that evaluating ESP is much more time-consuming than evaluating other functions. The ESP evaluated under default value is accurate enough in general cases. Reduced density gradient (RDG) RDG are a pair of very important functions for revealing weak interaction region96-98 for detail. RDG is defined as
96-98 . Fortunately, it is found that weak interaction analysis under pro-molecular density is still reasonable. Pro-molecular density is simply constructed by superposing electron densities of free-state atoms and hence can be evaluated extremely rapidly.
![]()
Where
![]()
Is pre-fitted spherically averaged electrons densities of atom A.
Result and Discussion
Thiswork basically focuses on the magnetic properties of CoFe2O4 in the non-bonded systems with B18N18 surfaces including “CoFe2O4@B18N18”. The CoFe2O4@B18N18nano-adsorbent shown high adsorption affinities for aqueousNa+, K+, Mg2+, and the amino-CoFe2O4@SiO2 exhibited high adsorbent for Fe (iii), Pb (ii), Ni (ii), Cu (ii) and Zn (ii) ions, resulting from complexation of the metals ion with the surface amino groups.The metalloaded CoFe2O4@B18N18 nanoparticles could be recovered easily from aqueous solution bymagneticseparation. The data have shown in ten figures and eight tables.As it is indicated in table1, LOL is low and constant for Both CoFe2O4@silica, CoFe2O4@B18N18 and CoFe2O4@ B18N18.ELF has a similar expression asLOL.
The non-bonded interactions are shown in figs1-3.As it is indicated in tables 1-4, the electrical properties can be obtained from changes in the non-bonded interactions. Potential energy densities, ELF, LOL,electron densities, energy densities,eta index and ECP are shown in tables1-4. The results of ELF and LOL indicate that the surfaces of silica and B18N18are suitable to attach in aromatic and organic compounds in any scale from nano to micro or medium.
Table 1: All Electron Densities of non-bonded interactions for CoFe2O4@Silica shell (a),CoFe2O4@B18N18 shell (b) andCoFe2O4 (isolate)C
| Atoms of CoFe2O4 | Density of all electron (10-3)(a) (c) (b) | Density of alpha(10-3)(a) (c) (b) | Density of Beta(10-3)(a) (c) (b) | Spin Density (a) (c) (b) |
| Co(1) | 0.30 0.12 0.36 | 0.15 0.06 0.18 | 0.15 0.06 0.18 | 0.0 0.1 0.0 |
| Fe(2) | 0.28 0.10 0.34 | 0.14 0.05 0.17 | 0.14 0.05 0.17 | 0.0 0.1 0.0 |
| Fe(3) | 0.14 0.12 0.32 | 0.07 0.06 0.16 | 0.07 0.06 0.16 | 0.0 0.1 0.0 |
| O(1) | 0.32 0.16 0.14 | 0.16 0.08 0.07 | 0.16 0.08 0.07 | 0.0 0.0 0.0 |
| O(2) | 0.18 0.12 0.24 | 0.09 0.06 0.12 | 0.09 0.06 0.12 | 0.0 0.0 0.0 |
| O(3) | 0.30 0.18 0.12 | 0.15 0.09 0.06 | 0.15 0.09 0.06 | 0.0 0.0 0.0 |
| O(4) | 0.12 0.18 0.22 | 0.06 0.09 0.11 | 0.06 0.09 0.11 | 0.0 0.0 0.0 |
 |
Figure 1: the non-bonded interaction between CoFe2O4 and B18N18 shell |
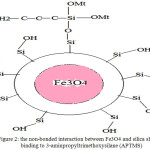 |
Figure 2: the non-bonded interaction between Fe3O4 and silica shell for binding to 3-aminpropyltrimethoxysilane (APTMS) Click here to View figure |
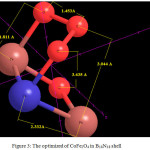 |
Figure 3: The optimized of CoFe2O4 in B18N18 shell Click here to View figure |
As it is shown in Figs.5-9, there are clear curves within a decreasing amount for electron density, energy density, ELF and LOL. The interaction energy between two sides of CoFe2O4-silica and CoFe2O4-B18N18 are also calculated. The potential energy difference between the two parts, are depicted in table 2.
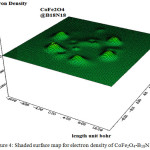 |
Figure 4: Shaded surface map for electron density of CoFe2O4-B18N18 shell Click here to View figure |
Table 2: All Electron Energies of non-bonded interactions for CoFe2O4@Silica shell (a),CoFe2O4@B18N18 shell(b) and CoFe2O4 (isolate)C
|
Atoms of CoFe2O4 |
Lagrangian kinetic [G(r)]energy (10-3) (a) (c) (b) | Hamiltonian kinetic [K(r)]energy (10-3)(a) (c) (b) | Potential energy Density [U(r)](10-3)(a) (c) (b) |
| Co(1) | 0.32 0.14 0.30 | 0.60 0.58 0.54 | -0.68 -0.52 -0.78 |
| Fe(2) | 0.33 0.12 0.31 | 0.62 0.66 0.62 | -0.65 -0.64 -0.63 |
| Fe(3) | 0.23 0.16 0.30 | 0.38 0.72 0.68 | -0.34 -0.37 -0.58 |
| O(1) | 0.28 0.21 0.14 | -0.32 -0.51 -0.43 | -0.24 -0.59 -0.50 |
| O(2) | 0.24 0.15 0.26 | -0.14 -0.40 -0.46 | -0.86 -0.68 -0.54 |
| O(3) | 0.16 0.15 0.18 | -0.44 -0.39 -0.44 | -0.33 -0.63 -0.55 |
| O(4) | 0.24 0.16 0.18 | -0.14 -0.42 -0.42 |
-0.85 -0.63 -0.51 |
Table 3: Laplacian, ELF, LOL and Local information entropy of non-bonded interactions for CoFe2O4@Silica shell (a),CoFe2O4@B18N18 shell (b)and CoFe2O4 (isolate)C
| Atoms ofCoFe2O4 | Laplacian of electron density (10-3)(a) (c) (b) | Electron localization function (ELF) (10-3)(a) (c) (b) | Localized orbital locator (LOL) (10-3)(a) (c) (b) | Local information entropy (10-3)(a) (c) (b) |
| Co(1) | -0.20 -0.26 -0.22 | 0.41 0.46 0.65 | 0.14 0.22 0.13 | 0.14 0.15 0.25 |
| Fe(2) | -0.25 -0.24 -0.24 | 0.41 0.48 0.66 | 0.12 0.24 0.14 | 0.20 0.34 0.25 |
| Fe(3) | -0.13 -0.27 -0.26 | 0.13 0.25 0.61 | 0.63 0.12 0.17 | 0.49 0.16 0.18 |
| O(1) | 0.79 -0.67 -0.55 | 0.13 0.17 0.25 | 0.32 0.25 0.25 | 0.13 0.12 0.10 |
| O(2) | 0.18 0.25 0.30 | 0.13 0.16 0.2 2 | 0.10 0.15 0.25 | 0.23 0.32 0.30 |
| O(3) | 0.84 0.14 0.25 | 0.16 0.15 0.26 | 0.31 0.12 0.25 | 0.32 0.20 0.31 |
| O(4) | 0.23 0.24 0.15 | 0.16 0.13 0.29 | 0.16 0.17 0.21 | 0.32 0.24 0.14 |
Table 4: Average local ionization energy and RDG of non-bonded interactions for CoFe2O4@Silica shell (a),CoFe2O4@B18N18 shell (b)and CoFe2O4 (isolate)C
|
Atoms of CoFe2O4 |
Reduced density gradient RDG) (10-3) |
Average local ionization energy |
|
(a) (c) (b) |
(a) (c) (b) |
|
|
Co(1) |
0. 4 0.5 0.5 |
0.4 0.4 0.5 |
|
Fe(2) |
0.3 0.4 0.5 |
0.6 0.5 0.5 |
|
Fe(3) |
0.4 0.5 0.5 |
0.4 0.4 0.5 |
|
O(1) |
0.6 0.4 0.6 |
0.7 0.6 0.4 |
|
O(2) |
0.3 0.4 0.5 |
0.3 0.5 0.4 |
|
O(3) |
0.6 0.3 0.6 |
0.7 0.3 0.5 |
|
O(4) |
0.4 0.3 0.5 |
0.3 0.4 0.5 |
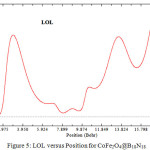 |
Figure 5: LOL versus Position for CoFe2O4@B18N18 |
It has been exhibited the BN compounds such as B18N18 and amino-functionalized materials presented an ability for removing a wide range of earth/alkali. In contrast, the most usual used magnetic sorbents are based on iron oxide and unfortunately thussusceptible to leaching under acidic conditions. This problem will removed by replacing the B18N18 instead of Sio2 as a shell of CoFe2O4. In addition, functional groups on the coating layer chemically adhering to the MNPs are also assailable to acid treatment.In contrast of SiO2 rings which is stable under acidic conditions the B18N18 rings is independent from acidic situations and hence for B18N18 comparing to SiO2, the functions is not needed as an ideal shell composite to protect the inner magnetite core. Although CoFe2O4@SiO2 or Silica-coated core–shell magnetite nanoparticles have recently been investigated for potentialbiomedical applications, by this work we exhibit the B18N18 is much useful for removing the earth/alkali metal from the aqueous solution.
Relative adsorption energies of four alkali and alkali/metal ions on CoFe2O4@B18N18have investigated. The adsorption data for Cu, Pb, Cd and so on were fitted to the Langmuir model according to the equation:
![]()
where Ce (mmol/L) and qe (mmol/g) and are the aqueous concentration the adsorbed concentration at equilibrium adsorption, and, b and qm are the coefficient of affinity and the adsorption capacity respectively101.
Conclusion
We think that B18N18 capabilities of magnetic substrates should be explored in the near future. Another interesting development is using the B18N18 on magnetic-nanoparticle enables effective removal of alkali -earth/alkali metals based on catalysts froms important pharmaceutical products in the drug nanotechnology. Our Calculations indicate that the B18N18 are suitable surfaces for CoFe2O4 such silica surfaces for removal metal ions such as Na+, Mg++,K+ and Ca++.
References
- Haneda, K.; Morrish, A.H. J. Appl. Phys1998, 63 (8) 4258.
CrossRef - Moumen,N.; Veillet, P. ; Pileni, M.P. J. Magn. Magn.Mater.1995,149 , 67.
CrossRef - Blaskov, V.; Petkov, V.; Rusanov, V.; Martinez, L.M.; Martinez, B.; Munoz, J.S.; Mikhov, M. J. Magn. Magn1996, Mater. 162,331.
- Pillai, V.; Shah, D.O.; J. Magn.Magn.Mater1996, 163,243
CrossRef - Davis, K.J.; Wells, S.; Upadhyay, S.V.; Charles, S.W.; O’Grady, K.M.; El Hilo, M.; Meaz, T.; Morup, S. J. Magn. Magn.Mater.1995, 149 14
CrossRef - Ma, Ming; Zhang, Yu; Guo, Zhir4.ui; GU, Ning. Nanoscale Research Letters2013, 8 (1): 16. doi:10.1186/1556-276X-8-16.
CrossRef - Massart, R., IEEE transactions on magnetics, 1981, 17, 2, 1247–1248
CrossRef - ValterStröm, Richard T. Olsson, K. V. Rao, J. Mater. Chem., 2010, 20, 4168-4175
- Shi, M.; Zuo, R. Z.; Xu, Y. D.; Jiang, Y. Z.; Yu, G. Y.; Su, H. L.; Zhong, J. G. J. Alloy. Compd. 2012, 512, 165170
CrossRef - Cui, H. T.; Jia, Y. Y.; Ren, W. Z.; Wang, W. H.J. Sol-Gel Sci. Technol. 2010, 55, 3640
CrossRef - Burianova, S.; Vejpravova, J. P.; Holec, P.; Plocek , J.; Niznansky, D.; J. Appl. Phys, 2011, 110,073902.
CrossRef - Zhu, Z. G.; Li, X. Y.; Zhao, Q. D.; Shi, Y.; Li, H.; Chen, G. H.; J. Nanopart. Res, 2011, 13 21472155.
- Zhang, Y.; Liu, Y.; Yang, Z.; Xiong , R.; Shi, J.; J. Nano-part. Res, 2011, 13, 45574563
- Peng, J. H.; Hojamberdiev, M.; Xu, Y. H.; Cao, B. W.; Wang, J.; Wu, H.; J. Magn. Magn.Mater.2011, 323, 133137
- Yanase, A.; Siratori, K.; J. Phys. Soc. Jpn.1984, 53, 312
CrossRef - Pe´nicaud, M.; Siberchicot, B.; Sommers, C. B.; Ku¨bler, J. Magn.Magn. Mater, 1992,103, 212
- Babes, L.; Denizot, B.; Tanguy, G.; Le Jeune, J.J.; Jallet P. Journal of Colloid and Interface Science , 1999, 212 (2): 474–482
CrossRef - BERRY, C.; CURTIS, A S G. J Phys D: ApplPhys, 2003, 36: 198−206.
- RUUGE, E K.; RUSETSKI, A N. J MagnMagn Mater, 1993, 122, 335−339
- POPE, N M.; ALSOP, R C.; CHANG, Y A.; SMITH, A K. J Biomed Mater Res, 1994, 2: 449−457.
- Tago, T.; Hatsuta, T.; Miyajima, K.; Kishida, M.; Tashiro, S.; Wakabayashi, K.; Journal of the American Ceramic Society, 2002, 85, 9, 2188-2194.
CrossRef - Lei, Z.; Li, Y. L.; Wei, X. Y. Journal of Solid State Chemistry, 2008, 181, 3, 480-486.
CrossRef - Huang, C. Z. Hu, B.; SpectrochimActa Part B: Atomic Spectroscopy, 2008, 63, 437-444.
CrossRef - Philipse, A.P. J. Chem. Educ. 2011, 88, 59–62.
CrossRef - Sheldon, R.A.; Downing, R.S. Appl. Catal. A 1999, 189, 163–183.
CrossRef - Cole-Hamilton, D.J. Homogeneous catalysis—New approaches to catalyst separation, recovery, and recycling. Science 2003, 299, 1702–1706.
CrossRef - Cornils, B.; Herrmann, W.A. J. Catal.2003, 216, 23–31
CrossRef - Johnson, B.G. Nanoparticles in catalysis. Top.Catal.2003, 24, 147–159.
CrossRef - Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Chem. Rev. 2008, 108, 2064–2110.
CrossRef - Kodama, R.H. Magnetic nanoparticles. J. Magn. Magn.Mater.1999, 200, 359–372.
CrossRef - Chang, L.L.; Erathodiyil, N.; Ying, J.Y. Acc. Chem. Res. 2012, 46, 1825–1837.
CrossRef - Fujishima, A.; Zhang, X.; Tryk, D.A. Surf. Sci. Rep. 2008, 63, 515–582.
CrossRef - Wang, X.; Starz-Gaiano, M.; Bridges, T.; Montell, D. Protoc, Exch, 28, 2008.
- Wei, S.; Wang, Q.; Zhu, J.; Sun, L.; Lin, H.; Guo, Z. Nanoscale2011, 3, 4474–4502.
CrossRef - Yoon, T.-J.; Lee, W.; Oh, Y.-S.; Lee, J.-K. New J. Chem. 2003, 27, 227–229.
CrossRef - Yu, Zh.; Hu, M. L.; Zhang, C. X.; He, C. Y.; Sun, L. Z.; Zhong, J. J. Phys. Chem. C2011, 115, 10836.
CrossRef - Seifert, G.; Flower, P. W.; Mitchell, D.; Porezag, D.;Frauenheim, T. Chem. Phys. Lett.1997, 268, 352.
CrossRef - Alexandre, S. S.; Chacham, H.; Nunes, R. W. Appl. Phys. Lett.1999, 75, 61.
CrossRef - Locke, I. W.; Darwish, A. D.; Kroto, H. W.; Prassides, K.;Taylor, R.; Walton, D. R. M. Chem. Phys. Lett.1994, 225, 186.
CrossRef - Behrman, E. C.; Foehrweiser, R. K.; Myers, J. R.; French, B. R.;Zandler, M. E. Phys. Rev. A 1994, 49, R1543.
CrossRef - Kaxiras, E.; Jackson, K.; Pederson, M. R. Chem. Phys. Lett.1994,225, 448
CrossRef - Zhao, J. X.; Ding, Y. H. J. Phys. Chem. C 2008, 112, 5778.
CrossRef - Bagheri,S.; Moosavi,M.S..; Moradiyeh,N.; Zakeri,M.; Attarikhasraghi,N.; Saghayimarouf,N.; Niyatzadeh,G.; Shekarkhand,M.; Mohammad S. Khalilimofrad, Ahmadin,H.; Ahadi,M.; Molecules 2015, 20, 21636–21657;
- Strout, D. L. Chem. Phys. Lett. 2004, 383, 95.
CrossRef - Zope, R. R.; Dunlap, B. I. Chem. Phys. Lett. 2004, 386, 403.
CrossRef - Zope, R. R.; Baruah, T.; Pederson, M. R.; Dunlap, B. I. Chem.Phys. Lett.2004, 393, 300.
CrossRef - Zheng, J. W.; Zheng, L. P.; Wu, P. J. Phys. Chem. C 2010, 114,5792.
- Chopra, N. G.; Luyken, R. J.; Herrey, K.; Crespi, V. H.; Cohen, M. L.; Louie, S. G.; Zettl, A. Science1995, 269, 966.
- Blase, X.; Rubio, A.; Louie, S. G.; Cohen, M. L ́ Europhys. Lett.1994, 28L, 335.
- Monajjemi, M.; Lee, V.S.; Khaleghian, M.; B. Honarparvar, B.; F. Mollaamin, F. J. Phys.Chem C. 2010, 114, 15315
CrossRef - Monajjemi, M, Journal of Molecular Liquids, 2017, 230 , 461–472
CrossRef - Monajjemi,M.; Nayyer T. MohammadianJ. Comput. Theor.Nanosci.2015, 12, 4895-4914.
- Monajjemi, M.Struct Chem.2012, 23,551–580
CrossRef - Monajjemi, M.; Boggs, J.E. J. Phys. Chem. A, 2013,117,1670 −1684
CrossRef - Mollaamin, F.; Monajjemi, M, Journal of Computational and Theoretical Nanoscience. 2012, 9 (4) 597-601
CrossRef - Monajjemi, M.; Khaleghian, M, Journal of Cluster Science.2011, 22(4), 673-692318
CrossRef - Monajjemi, M.; Wayne Jr, Robert. Boggs, J.E. Chemical Physics.2014, 433, 1-11
- Monajjemi, M. Falahati, M.; Mollaamin, F.; Ionics, 2013, 19, 155–164
CrossRef - Monajjemi, M.; Mollaamin, F. Journal of Cluster Science, 2012, 23(2), 259-272
CrossRef - Tahan, A.; Monajjemi, M. Acta Biotheor,2011, 59, 291–312
CrossRef - Mollaamin, F.; Monajjemi, M.Physics and Chemistry of Liquids .2012, 50, 5, 2012, 596–604
- Monajjemi, M.; Khosravi, M.; Honarparvar, B.; Mollaamin, F.; International Journal of Quantum Chemistry, 2011, 111, 2771–2777
CrossRef - Monajjemi, M. TheorChemAcc, 2015, 134:77 DOI 10.1007/s00214-015-1668-9
CrossRef - Monajjemi, M. Journal of Molecular Modeling, 2014, 20, 2507
CrossRef - Monajjemi, M.; Khaleghian, M.; Mollaamin, F. Molecular Simulation. 2010, 36, 11, 865–
- Monajjemi, M. Biophysical Chemistry.2015.,207,114 –127
CrossRef - Jalilian,H.; Monajjemi, M. Japanese Journal of Applied Physics. 2015, 54, 8, 08510
- S. Shin, J. Jang, Chem. Commun.2007., 41., 4230.
CrossRef - L.C.A. Oliveira, D.I. Petkowicz, A. Smaniotto, S.B.C. Pergher, Water Res. 2004., 383699.
- J. Hu, G. Chen, I.M.C. Lo, Water Res.2005.,39., 4528.
CrossRef - C.T. Yavuz, J.T. Mayo, W.W. Yu, A. Prakash, J.C. Falkner, S. Yean, L. Cong, H.J. Shipley, A. Kan, M. Tomson, D. Natelson, V.L. Colvin, Science 2006.,314 964
CrossRef - B. Hai, J. Wu, X. Chen, J.D. Protasiewicz, D.A. Scherson, Langmuir2005.,213104.
- J. Hu, M.C. Lo, G.H. Chen, Sep. Purif. Technol. 2007.,58 .,76.
CrossRef - Y.C. Chang, D.H. Chen, J. Colloid Interface Sci. 2005., 283 446.
CrossRef - J.F. Liu, Z.S. Zhao, G.B. Jiang, Environ. Sci. Technol.2008., 42 ., 6949.
CrossRef - W. Yantasee, C.L. Warner, T. Sangvanich, R.S. Addleman, T.G. Carter, R.J. Wiacek, G.E. Fryxell, C. Timchalk, M.G. Warner, Environ. Sci. Technol.2007., 415114.
- M. Kumar, D.P.S. Rathore, A.K. Singh, Talanta2000.,51., 1187.
CrossRef - K.F. Lam, K.L. Yeung, G. McKay, Environ. Sci. Technol.2007., 41.,3329.
CrossRef - J. Li, X. Miao, Y. Hao, J. Zhao, X. Sun, L. Wang, J. Colloid Interface Sci. 2008.,.318 309.
CrossRef - Q. Liu, Z. Xu, J.A. Finch, R. Egerton, Chem. Mater. 1998.,10 .,3936.
CrossRef - C.W. Lu, Y. Hung, J.K. Hsiao, M. Yao, T.H. Chung, Y.S. Lin, S.H. Wu, S.C. Hsu, H.M. Liu, C.Y. Mou, C.S. Yang, D.M. Huang, Y.C. Chen, Nano Lett. 2007.,7 .,149.
CrossRef - P. Ashtari, X.X. He, K. Wang, P. Gong, Talanta.,2005.,67 548.
CrossRef - Zhao, Y.; Truhlar, D.G. TheorChemAccount ,2008, 120:215–241, DOI 10.1007/s00214-007-0310-x
CrossRef - Zhao, Y.; Truhlar, D.G, Accounts of Chemical Research.2008, 41, (2): 157-167.
CrossRef - Monajemi, M; Ketabi, S; Zadeh, MH; et al. Biochemistry-Moscow 2006, 71 S1-S8
CrossRef - Grimme, S. AngewChemInt Ed, 2006, (45), 4460–4464, DOI: 10.1002/anie.200600448.
CrossRef - Schreiner, P.R.; Fokin, A. A.; Pascal, R. A Jr.; de Meijere, A. Org. Lett, 2006, (8): 3635–3638.
CrossRef - Zhao, Y.; Truhlar, D.G. Org. Lett, 2006, (8):5753–5755.
CrossRef - Kohn, W.; Sham, LJ. Phys. Rev,1965, (140) A: 1133-1138.
- Perdew, J .P. Burke, K.; Phys. Rev. Lett.1996, (77): 3865-3868.
CrossRef - Besler, B.H.; Merz, K.M.; Kollman, P.A. J. comp. Chem, 1990, (11): 431-439,DOI: 10.1002/jcc.540110404
CrossRef - Brneman, G.M, Wiberg, K.B. J. Comp Chem, 1990, (11): 361
- Chirlian, L.E.; Francl, M.M. J.comp.chem,1987, (8): 894-905, DOI: 10.1002/jcc.540080616
CrossRef - Martin, F.; Zipse, H. J Comp Chem. 2005, (26): 97 – 105.
- Balderchi, A.; Baroni, S.; Resta, R. Phys. Rev. Lett, 1998, (61): 173.
- Lu, T.; Chen, F. ActaChim. Sinica, 2011, 69, 2393-2406.
- Lu, T.; Chen, F J. Mol. Graph. Model, 2012, (38): 314-323.
CrossRef - Lu, T.; Chen F. J. Comp. Chem. 2012, (33) 580-592.
- Schmider and Becke, J. Mol. Struct. (THEOCHEM), 527, 51
- Murray and coworkers, J. Mol. Struct. (THEOCHEM), 307, 55
- Jiahong Wang, Journal of Colloid and Interface Science 2010.,349., 293–299
CrossRef - Mollaamin, F.; Baei, M. T.; et al.Russian Journal of Physical Chemistry A, (2008)82 ,13,2354-2361
- Mollaamin,Fatemeh; Journal of Computational and Theoretical nanoscience 2012597-601
- Mollaamin, F.; Varmaghani, Z.; Physics and Chemistry of Liquids 201149 ,3 318- 336

This work is licensed under a Creative Commons Attribution 4.0 International License.









