Study of the Corrosion Resistance of Type 304L and 316 Austenitic Stainless Steels in Acid Chloride Solution
Roland Tolulope Loto1, 2
1Department of Mechanical Engineering, Covenant University, Ota, Ogun State, Nigeria.
2Department of Chemical Metallurgical and Materials Engineering, Tshwane University of Technology, Pretoria, South Africa.
Corresponding Author E-mail: tolu.loto@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330304
The corrosion resistance of type 304L and 316 austenitic stainless steels in 2M H2SO4 at 0-1.5%NaCl concentrations was studied through potentiodynamic polarization technique and optical microscopy analysis. The corrosion rate, pitting potential, passivation potential and surface morphology of both steel where significantly altered by the Cl- ion concentration, alloy composition and metallurgical properties of the steels. Results showed that 316 stainless steel significantly performed better than the 304L counterpart with the unusual phenomenon of decreasing corrosion rate with increase in Cl- ion concentration. 304L steel showed no passivation and resistance to pitting after 0% NaCl concentration coupled with increase in corrosion rate. Despite similar elemental composition, the presence of molybdenum had a strong influence on the corrosion resistance and passivation of 316 steel. The surface morphology of 316 steel showed mild deterioration compared to severe surface deterioration, and visible micro/macro-pits on 304L.
KEYWORDS:Corrosion; pitting; passivation; steel
Download this article as:| Copy the following to cite this article: Loto R. T. Study of the Corrosion Resistance of Type 304L and 316 Austenitic Stainless Steels in Acid Chloride Solution. Orient J Chem 2017;33(3). |
| Copy the following to cite this URL: Loto R. T. Study of the Corrosion Resistance of Type 304L and 316 Austenitic Stainless Steels in Acid Chloride Solution. Orient J Chem 2017;33(3). Available from: http://www.orientjchem.org/?p=33474 |
Introduction
Seawater environments are extensively used by industries such as shipping, offshore oil and gas production, power plants and coastal industrial plants mainly for cooling, oil field water injection and for desalination plants. Corrosion problems in these environments have been researched into but failures still occur. The basic cause of metallic corrosion is the inherent instability of metal alloys in their refined forms as they tend to revert to their natural states through the processes of corrosion. The major cause of corrosion in these environments is the presence of chloride anions. Cost of corrosion damage in the maritime industry as a result of the routine exposure of vessels and marine structures to these chloride containing environments has increased geometrically every year with an estimated total cost of between $50-80 billion worldwide with the oil and gas taking a significant chunk of the cost1, 2. Petrochemical structures routinely apply stainless steels tubing in process instrumentation and sensing, chemical inhibition, hydraulic lines, impulse lines, and utility applications, over an extensive variation of temperatures, flows, and pressures. The corrosion resistance and electrochemical behaviour of stainless steels in seawater is of great interest due to their extensive application therein. The corrosive nature of the marine environment has a strong influence on the passivation characteristics of the steel.Once the passive film weakens and breakdown, an electrochemical cell initiates whereby the substrate Iron oxidizes to iron oxide. The consequences are accelerated pitting, perforation, leaks and collapse of material structures and equipment’s. Pitting can penetrate deep into the tubing walls, creating a situation where tubing could fail3. A number of studies have been performed on passive films behavior of stainless steels in aqueous solutionscontaining chloride or neutral free chloride solutions, but however the optimal condition under which the steels can perform effectively without corroding has not been established4-6. As end products and industrial processes have become more costly, complex and under scrutiny from regulatory authorities, the consequences of failures from corrosion, including safety hazards and breakdown in plant operations, have become more costly and more specifically recognized. The focus given to the control and prevention of corrosion has increased with strong emphasis on material selection7. Some metals are more resistant to corrosion than others, probably as a result of the fundamental property of the electrochemical processes involved or due to the nature of the corrosive environment. Stainless steels must be applied in environments where they will perform more effectively. The corrosion resistance of stainless steels depends on various metallurgical and processing variables. Austenitic grade is considered to be most resistant to industrial atmospheres including aggressive aqueous and non-aqueous acid media8, 9. Type 304L stainless steel is an extra low-carbon variation of Type 304 with 0.03% maximum carbon content. The alloy with 316 stainless steel can be used in severe corrosive conditions as it performs well in fresh water service with limited levels of chlorides, and in many organic and inorganic chemicals in moderately oxidizing to moderately reducing environments10, 11. This research aims to study and compare the corrosion behavior, pitting corrosion resistance and passivation characteristics of 304L and 316 austenitic stainless steels in mild chloride environments.
Materials and Methods
Materials and Preparation
304L austenitic stainless steel (304LSS) and 316 austenitic stainless steel (316SS) sourced commercially had a nominal composition as shown in Table 1. The steel electrodes after mounting in epoxy resin according to ASTM G59-97(2014)12 have an exposed surface area of 0.79cm2 and 1.33cm2 respectively. The steel specimens after machining were abraded with silicon carbide papers before washing with distilled water and propanone for potentiodynamic polarization test according to ASTM G1 – 03(2011)13. Recrystallized NaCl obtained from Titan Biotech, India was prepared in volumetric concentrations of 0.25%, 0.5%, 0.75%, 1%, 1.25% and 1.5%in 200 mL of 2M H2SO4 solution, prepared from analar grade of H2SO4 acid (98%) with deionized water. Polarization test was carried out at 30◦C with a three electrode system and glass cell with the electrolyte using Digi-Ivy 2311 potentiostat. Plots were obtained at a scan rate of 0.0015V/s between potentials of −0.5V and +1.5V according to ASTM G102-89(2015)14. Corrosion current density (Jcr, A/cm2) and corrosion potential (Ecr, V) values were obtained using the Tafel extrapolation method. The corrosion rate (CR) was calculated from the mathematical relationship;

where Eqv is the sample equivalent weight in grams. 0.00327 is a constant for corrosion rate calculation in mm/y15.
Table 1: Percentage Nominal Composition of 304LSS and 316SS
| Element Symbol | Si | N | Ni | Mo | Cr | Mn | P | S | C | Fe |
| % Composition (304LSS) | 0.75 | 0.1 | 10 | – | 18 | 2 | 0.045 | 0.03 | 0.03 | 69.31 |
| % Composition (316SS) | 0.75 | 0.1 | 11 | 3 | 18 | 2 | 0.045 | 0.03 | 0.08 | 65 |
Optical Microscopy Characterization
Images of control and corroded 304LSSSS and 316SS surface morphology from optical microscopy were analysed after weight-loss measurement with Omax trinocular metallurgical microscope through the aid of ToupCam analytical software.
Result and Discussion
Potentiodynamic Polarization Studies
The corrosion polarization behaviour of 304LSS and 316SS samples in 2M H2SO4 acid media at 0-1.5% NaCl concentration is shown in Figs. 1 & 2. Table 2 shows the results from the potentiodynamic polarization plots. It can be observed that the values for 304LSS increased with increase in NaCl concentration as compared to 316SS which showed an unusual phenomenon whereby its corrosion rate decreased significantly with increase in NaCl concentration accompanied by a proportionate decrease in corrosion current density probably due to the presence of molybdenum in its metallurgical composition. 316SS is more likely to survive in marine environments as the oxidizing strength of the H2SO4/NaCl decreases in the presence of 316SS contrary to its electrochemical performance in the presence of 304LSS. These observations are further confirmed from the corrosion potential values of both steels. 304LSS has higher corrosion potential values than 316SS, thus 316SS is less likely to polarize in the under similar conditions with 304LSS. Based on the Lewis acid-base concept, the surface of 304LSS more easily forms soft acid due to adsorption of Cl– ions leading to accelerated corrosion in comparison to 316SS16. This is responsible for the breakdown of 304LSS passive film after 0%NaCl resulting in localized corrosion of the substrate metals. The lower corrosion potential exhibited by 316SS at lower corrosion rates suggests that the passive film thickness of the steel is much higher than 304LSS. This is responsible for the passivation behaviour as it takes a longer time for the steel to locally dissolve and thin out before the underlying metal begins to corrode17, 18.
304LSS underwent limited pitting corrosion resistance in the acid chloride media. Potentiostatic values presented in Table 3 shows that after 0%NaCl concentration, the pitting corrosion resistance declined drastically to insignificant values. Limited passivation behaviour can be observed on the polarization plot however they are too negligible to make any significant difference on the passivation characteristics and pitting corrosion resistance of the stainless steel alloy. The pitting and passivation potential values for 316SS (Table 3) shows a material with unusual properties. It can be seen that in addition to the property of decreasing corrosion rate with increase in NaCl concentration, the pitting potential also increased marginally with increase in NaCl concentration till 1%NaCl. The passivation potential and current at passivation potential decreased with increase in NaCl concentration due to the electrolytic action of Cl– ions which delayed the passivation (protection ability) of the steel as a result of metastable pitting activity. in the metastable region of the polarization plots, an indication that the passive film is undergoing localized but transient pitting due to temporary breakdown of the passive film, and the creation and growth of small, occluded cavities before stable passivation. 316SS was unable to passivate after 1%NaCl (1.25% and 1.5%NaCl). 316SS displayed stronger resistance to pit and general corrosion thus is more liable to survive for longer periods in marine environments. Studying the marginal change in pitting corrosion potential of 316SS with respect to visible changes in its passivation potential, it is suggested that Cl– ion concentration has limited negative influence on the pitting corrosion characteristics of the steel in comparison to its passivation behaviour. The Cl- tends to hinder the formation of the passive film with increase in concentration reducing the passivation range and strength of the passive film before the onset of pitting corrosion.
It must be noted that elemental composition is one of the key factors responsible for the wide contrast in electrochemical behaviour and passivation characteristics both steels. Observation of Table 1 shows that both steels have the same elemental composition but with the exception of Mo in 304LSS. Molybdenum is an important alloy element, which is widely used in metallurgy. It has been known to improve the corrosion resistance of stainless steels alloys and steels containing this element tend to be more resistant than molybdenum-free grades19, 20. Molybdenum has been known to be a ferrite former which in the presence of manganese and nickel it keeps the structure of 316SS austenitic21. In H2SO4/NaCl media it enriches Cr and Mo at the metal/solution interface which stabilizes and thickens the passive film of 316SS22-27.
Table 2: Polarization results for 304LSS in 2M H2SO4 at 0-1.5%NaCl
| Sample | 2M H2SO4/NaCl Conc. (%) | Corrosion Rate (mm/y) | Corrosion Current (A) | Corrosion Current Density (A/cm2) | Corrosion Potential (V) | Polarization Resistance, Rp | Cathodic Tafel Slope (Bc) | Anodic Tafel Slope (Ba) |
| A | 0 | 26.97 | 2.08E-03 | 2.63E-03 | -0.310 | 12.38 | -9.736 | 2.066 |
| B | 0.25 | 29.05 | 2.24E-03 | 2.83E-03 | -0.323 | 11.50 | -9.886 | -1.094 |
| C | 0.5 | 30.04 | 2.31E-03 | 2.93E-03 | -0.313 | 15.95 | -11.03 | 4.007 |
| D | 0.75 | 32.22 | 2.48E-03 | 3.14E-03 | -0.328 | 14.35 | -9.887 | 0.230 |
| E | 1 | 34.23 | 2.63E-03 | 3.33E-03 | -0.322 | 13.28 | -9.412 | 2.050 |
| F | 1.25 | 35.51 | 2.73E-03 | 3.46E-03 | -0.338 | 9.41 | -9.671 | 0.000 |
| G | 1.5 | 35.57 | 2.74E-03 | 3.46E-03 | -0.343 | 9.387 | -8.689 | 0.000 |
Table 3: Polarization results for 316SS in 2M H2SO4 at 0-1.5%NaCl
| Sample | 2M H2SO4/NaCl Conc. (%) | Corrosion Rate (mm/y) | Corrosion Current (A) | Corrosion Current Density (A/cm2) | Corrosion Potential (V) | Polarization Resistance, Rp | Cathodic Tafel Slope (Bc) | Anodic Tafel Slope (Ba) |
| A | 0 | 37.908 | 4.83E-03 | 3.63E-03 | -0.253 | 5.32 | 0.000 | -6.354 |
| B | 0.25 | 25.973 | 3.31E-03 | 2.49E-03 | -0.300 | 8.69 | 0.241 | 0.126 |
| C | 0.5 | 24.066 | 3.07E-03 | 2.31E-03 | -0.310 | 9.01 | -0.332 | 0.813 |
| D | 0.75 | 23.331 | 2.97E-03 | 2.24E-03 | -0.310 | 9.26 | -0.282 | 1.051 |
| E | 1 | 19.978 | 2.55E-03 | 1.91E-03 | -0.287 | 10.09 | -8.357 | 0.000 |
| F | 1.25 | 18.040 | 2.30E-03 | 1.73E-03 | -0.319 | 12.28 | -8.490 | 0.207 |
| G | 1.5 | 14.728 | 1.88E-03 | 1.41E-03 | -0.321 | 13.69 | -8.261 | 0.728 |
Table 4: Potentiostatic data of pitting and passivation potentials for 304LSS and 316SS in 2M H2SO4 solution at 0-1.5%NaCl
| Sample | 2M H2SO4/NaCl Conc. (%) | Pitting Potential, Epitt (V) | Current at Epitt (A) | Passivation Potential, Epp (V) | Current at Passivation Potential, Epp (A) |
| 304LSS | |||||
| A | 0 | 1.08 | 1.40E-03 | -0.19 | 7.39E-03 |
| 316SS | |||||
| A | 0 | 1.04 | 9.93E-05 | -0.27 | 3.31E-03 |
| B | 0.25 | 1.06 | 6.76E-05 | -0.25 | 4.82E-03 |
| C | 0.5 | 1.05 | 8.12E-05 | -0.24 | 7.28E-03 |
| D | 0.75 | 1.06 | 5.28E-05 | -0.23 | 7.65E-03 |
| E | 1 | 1.05 | 1.04E-04 | -0.06 | 7.65E-03 |
| F | 1.25 | 0 | 0 | 0 | 0 |
| G | 1.5 | 0 | 0 | 0 | 0 |
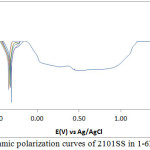 |
Figure 1: Potentiodynamic polarization curves of 2101SS in 1-6M H2SO4 solutions |
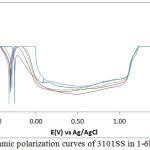 |
Figure 2: Potentiodynamic polarization curves of 3101SS in 1-6M H2SO4 solutions |
Optical Microscopy Analysis
The optical microscopy images of 304LSS and 316SS before and after the corrosion test at 1.5% NaCl are shown from Fig. 4(a) to 5(b) at mag. x40. Fig 4(b) shows a severely deteriorated surface of 304LSS in comparison to 316SS [Fig. 5(b)] with visible micro and macro-pits due to the electrolytic action of Cl– ions. The morphology of 316SS shows an alloy with strong resistance to surface oxidation and pitting, though its morphology in comparison to Fig. 5(a) shows it has undergone mild surface deterioration. The visible pits on 304LSS resulted from an autocatalytic process. This localized dissolution of the alloy is one of the most common and catastrophic causes of failure of metallic structures. Generally the surface oxides of stainless steels are mainly composed of iron (III) and chromium (III) oxides in most acidic environments at ambient temperature. Comparing the morphologies of both steel, it can be deduced that the relative proportion of Cr to Fe in the surface oxide of 304SS was significantly altered upon contact with Cl– ions28-31. Oxidation of the substrate iron in the steel after destruction of its passive protective film caused an increase in localized acidity within the pits upon initiation due to the half-cell reaction between the pits which acts as the anode and the metal surface which acts as the cathode. This results in the diffusion of Cl– ions into the pit to maintain charge neutrality as a result of excess positive charges from the oxidized metal32. The presence of molybdenum among the elemental constituents of 316SS is a major contributing factor for the steel morphology after corrosion as it increases lattice strain, thus making the surface tougher and increasing the energy required to dissolve iron atoms from the surface.
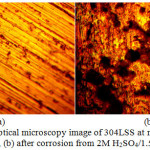 |
Figure 3: Optical microscopy image of 304LSS at mag. x40 (a) before corrosion, (b) after corrosion from 2M H2SO4/1.5% NaCl solution |
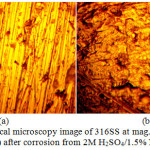 |
Figure 4: Optical microscopy image of 316SS at mag. x40 (a) before corrosion, (b) after corrosion from 2M H2SO4/1.5% NaCl solution |
Conclusion
Cl– ion concentration in 2M H2SO4 had a debilitating effect on the corrosion resistance and passivation characteristics 304LSS. The corrosion rate of the steel decreased with increase in Cl- ion concentration and its polarization behaviour showed no resistance to pitting corrosion from the lowest to the highest Cl– ion concentration due to the destruction of its passive film. Changes in Cl– ion concentration had positive influence on the electrochemical characteristics of 316SS. Increase in the oxidation strength of the corrosive test solution provoked a strong resistance to corrosion with decreasing corrosion rate as the Cl– ion concentration increased, however its pitting corrosion resistance decreased proportionately. The presence of molybdenum in the metallurgical structure of the steel had a significant influence on its corrosion behaviour. 316SS had a significantly better surface morphology compared to 304LSS due to the absence of micro/macro-pits and the morphological deterioration was relatively mild.
Acknowledgement
The author is grateful to Covenant University, Ota, Ogun State, Nigeria for the funding of the research and provision of research facilities.
References
- Corrosion in the maritime industry. http://www.nace.org/Corrosion-Central/Industries/Maritime-Industry/. [Retrieved: 30/03/2017].
- Marine Corrosion, http://www.marinecorrosionforum.org/explain.htm. [Retrieved: 30/03/2017].
- Gerhard, S.; Anibal, D.; Akinyemi, O.; Charlie, S. World Oil. 2009, 73-80.
- Landolt, D. Prococeedings on International Conference on Localized Corrosion, Orlando, U.S.A, 1987.
- Fischmeister, H.; Roll, U.; Fresenius, Z. Anal. Chem. 1984, 319: 639-645
CrossRef - Elsener, B.; DeFilippo, D.; Rossi, D. European Symposium on Modifications of Passive films, European Federation of Corrosion Publications, Institute of Materials, London, 1994, 12, p. 6.
- Strategic Impact and Cost of Corrosion Damage, http://www.corrosion-doctors.org/Why-Study/Strategic-Impact.htm. [Retrieved: 30/03/2017].
- Opiela, M.; Grajcar, A.; Krukiewicz, W. J. of Achievements in Mats. & Manuf. Eng. 2009, 33(2), 159-165.
- Surowska, B.; Weroski, A. Proceedings of the 14th International scientific conference, Advanced Materials and Technologies, Gliwice Zakopane, 1995, 425-428.
- 304/304L stainless steel.
- http://www.aksteel.com/pdf/markets_products/stainless/austenitic/304_304L_Stainless_Steel_DS_201512.pdf. [Retrieved: 30/03/2017].
- Stainless Steel – Grade 316 (UNS S31600). www.azom.com/article.aspx?ArticleID=863 [Retrieved: 30/03/2017].
- ASTM G59 – 97(2014), Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements. http://www.astm.org/Standards/G31 [Retrieved: 30/03/2017].
- ASTM G1 – 03(2011), Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. http://www.astm.org/Standards/G1 [Retrieved: 30/03/2017].
- ASTM G102 – 89(2015)e1. Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. http://www.astm.org/Standards/G31 [Retrieved: 30/03/2017].
- Choi, Y.; Nesic, S.; Ling, S. Electrochim. Acta. 2011, 56, 1752-1760.
CrossRef - Jensen, W.B. The Lewis Acid-Base Concepts, John Wiley & Sons, Inc., New York, 1980, 112–336.
- Fushimi, K.; Azumi, K.; Seo, M. J. Electrochem. Soc. 2000, 147(2), 552–557.
CrossRef - Heusler, K.E.; Fisher, L. Werkst. Korros. 1976, 27(8), 551–556.
CrossRef - International stainless steel forum, http://www.worldstainless.org/Files/issf/non-image-files/PDF/TheStainlessSteelFamily.pdf. [Retrieved: 30/03/2017].
- Metallurgy of Mo in stainless steel, International molybdenum association, http://www.imoa.info/molybdenum-uses/molybdenum-grade-stainless-steels/metallurgy-of-molybdenum-in-stainless-steel.php. [Retrieved: 30/03/2017].
- Hiramatsu, N.; Stott, F.H. Oxidation of Metals. 2000, 53(56), 561-576.
CrossRef - Abreu, C.M.; Cristo´bal, M.J.; Losada, R.; No´voa, X.R.; Pena, G.; Pe´rez, M.C. Electrochim Acta. 2004, 49(17-18), 3049–3056
CrossRef - Montemor, M.F.; Simo˜es, A.; Ferreira, M.G.S.; Da Cunha, M.B. Corros. Sci. 1999, 41(1), 17–34
CrossRef - Lu, Y,C.; Clayton, C.R. Corros. Sci. 1989, 29(8), 927–937
CrossRef - Vignal, V.; Olive, J.M.; Desjardins, D. Corros. Sci. 1999, 41(5), 869–884
CrossRef - Olefjord, I.; Brox, B.; Jelvestam, U. J. Electrochem. Soc. 1985, 132(12), 2854–2861
CrossRef - Schultze, J.W.; Lohrengel, M.M.; Ross, D. Electrochim Acta. 1983, 28(7), 973–984
CrossRef - Hedberg, Y.; Wang, X.; Hedberg, J.; Lundin, M.; Blomberg, E.; Odnevall Wallinder, I. J. Mater. Sci. Mater. M. 2013, 24(4),1015-1033
CrossRef - Olsson, C.O.A.; Landolt, D. Electrochim. Acta. 2003, 48(9), 1093-1104
CrossRef - Laoire, C.O.; Timmins, B.; Kremer, L.; Holmes, J.D.; Morris, M.A. Anal. Lett. 2006, 39(11), 2255-2271
CrossRef - Elsener, B.; Rossi, A. Mater. Sci. Forum. 1995, 192–194(1), 225-236
CrossRef - Lawrence, J. K.; ASM. Metals Handbook (ASM Handbook)- Corrosion, 9th Ed., 1987, 13

This work is licensed under a Creative Commons Attribution 4.0 International License.









