Design and Synthesis of New Dioxa-Diazaspiro[Bicyclo[9.4.2]Heptadecane-Steroid-Dienyne Derivative from Estrone and OTBS-Estrone
López-Ramos Maria1, Figueroa-Valverde Lauro1, Rosas-Nexticapa Marcela3, Herrera-Meza Maria Del Socorro4, Cervantes-Ortega Catalina3, Díaz-Cedillo Francisco2, García-Cervera Elodia1 and Pool-Gómez Eduardo1
1Laboratory of Investigation, Faculty Chemical-Biological Sciences; University Autonomous of Campeche. Agustin Megar s / n, Bellavista C.P. 24039, Campeche, Mexico.
2National School of Biological Sciences of the National Polytechnic Institute. Prol. Carpio and Plan de Ayala s / n Col. Santo Tomas, Mexico, D.F. C.P. 11340.
3Faculty of Nutrition, Universidad Veracruzana. Doctors and Dentists s / n, 91010, Xalapa Veracruz, Mexico.
4Instituto de Investigaciones Psicológicas. Veracruz University. Av. Dr Luis Castelazo S / N Col. Industrial Animas Xalapa Veracruz, Mexico.
Corresponding Author E-mail: lauro_1999@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/330301
Several bicycle-derivatives have been prepared using different protocols; nevertheless, expensive reagents and special conditions are required. The aim of this study was synthesize a dioxa-diazaspiro[bicyclo[9.4.2]heptadecane-steroid-dienyne derivative by a series of reactions which involving; a) alkinilization of estrone or OTBS-estrone with 5-hexyn-1-ol to form two propargyl alcohol derivatives (3 or 4); b) esterification 3 or 4 with succinic acid to form two dioxaspiro-steroid-cyclotridecan derivatives (5 or 6); c) preparation of diazaspiro[bicycle[9.4.2]heptadecane-steroid-4 amino complex (7 or 8) by reaction of 5 or 6 with ethylenediamine; d) removal of silyl fragment of 8 via hydrofluoric acid to form the compound diazaspiro[bicycle[9.4.2]heptadecane-steroid-3´-ol (9); e) preparation of diazaspiro[bicycle[9.4.2]heptadecane-steroid-4-oxobutanoic acid (10) via esterification of 9 with succinic acid; f) amidation of 10 with ethylenediamine to form diazaspiro[bicyclo[9.4.2]heptadecane-steroid-4-aminobutanoate (11); g) synthesis of dioxadiazaspiro [bicyclo[9.4.2]heptadecane-steroid-dienyne (12) via pyrrolization of 11 using boric acid. The chemical structure of compounds was confirmed by NMR spectroscopic data.
KEYWORDS:OTB-estrone; Chemical synthesis
Download this article as:| Copy the following to cite this article: Maria L, Lauro F, Marcela R, Socorro H. M. D, Catalina C, Francisco D, Elodia G, Eduardo P. Design and Synthesis of New Dioxa-Diazaspiro[Bicyclo[9.4.2]Heptadecane-Steroid-Dienyne Derivative from Estrone and OTBS-Estrone. Orient J Chem 2017;33(3). |
| Copy the following to cite this URL: Maria L, Lauro F, Marcela R, Socorro H. M. D, Catalina C, Francisco D, Elodia G, Eduardo P. Design and Synthesis of New Dioxa-Diazaspiro[Bicyclo[9.4.2]Heptadecane-Steroid-Dienyne Derivative from Estrone and OTBS-Estrone. Orient J Chem 2017;33(3). Available from: http://www.orientjchem.org/?p=33858 |
Introduction
Since several years ago, have been prepared several bicylo-derivatives which represent a very interesting class of compounds that have attracted the attention of many organic chemists. For example, the synthesis of 1,3-diace- tylbicyclo[l.l.l]pentane by the reaction of 1,1-dibromo-2,2-bis(chloromethyl)cyclopropane and methyllithium1. Other study, showed the selenium-mediated cyclization of alkenyl-substituted β-dicarbonyls (I) to form a variety of bicyclo[3.3.1]nonan-9-ones both in solution and on solid support2. In addition, other report indicated the preparation of an azabicyclo[2.2.l]heptane derivative by the reaction of N-Benzyl-5-[1′-(methoxycarbony1)-3′-oxobutyl] proline with oxalyl chloride-1,2-dichloro- ethane3. Also, a study showed the reaction of (E)-Hex-3-ene-1,6-dithiol with p-anisaldehyde to form an azathia-bicycle derivative4. Other data showed the coupling of cinnamaldehyde to (E)-hex-3-ene-1,6-ditosylamide in the presence of Sc(OTf)3 to form trans-fused octahydropyrrolo[3,2-c]pyridine5. Additionally, a study shown the preparation of bicycle [3.2.1]octan-8-one by the reaction of 1,2-cyclohexanedione with β-nitros-tyrene6. Other report indicate the preparation of 9-(Phenylthio)bicyclo[3.2.2]nona-3,6-dien-2-one by reac- tion of 2,4,6-cycloheptatrien-l-one with phenyl vinyl sulfide7. In addition, a study indicated the synthesis of 7,7-dichloro-cis-bicyclo[4.2.0]octan-8-one by reaction of cyclo- hexene with trichloroacetyl bromide/copper(II) sulfate8. All these experimental results show several procedures which are available for synthesis of diverse bicycle derivatives; nevertheless, expensive reagents and special conditions are required. Therefore, in this study a diazaspiro[bicyclo[9.4.2]heptadecane derivative from estrone and OTBS-estrone (Scheme 1) was synthetized using some chemical tools. It is noteworthy that this compound could be used in some biological model to evaluate their pharmacological activity for therapeutic purposes.
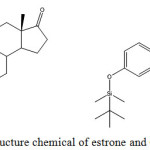 |
Scheme 1: Structure chemical of estrone and OTBS-estrone. |
Material and Methods
General Methods
The OTB-estrone (3-(Tert-butyl-dimethyl-silanyloxy)-13-methyl-6,7,8,9,11,12,13,14,15, 16-decahydro-cyclopenta[a] phenanthren-17-one) was prepared by a method previously reported9. The others reagents used in this study were purchased from Sigma-Aldrich Co. Ltd. The melting point was determined on an Electrothermal (900 model). Infrared spectra (IR) were recorded using KBr pellets on a Perkin Elmer Lambda 40 spectrometer. 1H and 13C NMR spectra were recorded on a Varian VXR-300/5 FT NMR spectrometer at 300 and 75.4 MHz in CDCl3 using TMS as internal standard. EIMS spectra were obtained with a Finnigan Trace GCPolaris Q. spectrometer. Elementary analysis data were acquired from a Perkin Elmer Ser. II CHNS/0 2400 elemental analyzer.
Chemical Synthesis
Preparation of two Propargyl Alcohol Derivatives (3 or 4)
A mixture of estrone or OTBS-estrone (0.50 mmol), 5-hexyn-1-ol (60 µl, 0.54 mmol), potassium hydroxide (30 mg, 0.53 mmol), in 5ml of methanol was stirring for 72 h to room temperature. The reaction mixture was evaporated to dryness under reduced pressure. After, the residue was purified by crystallization from methanol:water (4:1)
17-(6-Hydroxy-Hex-1-Ynyl)-13-Methyl-7,8,9,11,12,13,14,15,16,17-Decahydro-6H-Cyclo Penta[A]Phenanthrene-3,17-Diol
yielding 55 % of product, m.p. 182-184oC; IR (Vmax, cm-1): 3400 and 2192; 1H NMR (300 MHz, CDCl3) δH: 0.92 (s, 3H), 1.22-1.50 (m,4H), 1.58-1.60 (m, 4H), 1.70-2.10 (m, 6H), 2.20 (t, 2H, J = 13.47 Hz), 2.24- 2.76 (m, 5H), 3.66 (t, 2H, J = 11.00 Hz), 5.54 (broad, 3H), 6.48-7.10 (m, 3H) ppm. 13C NMR (75.4 Hz, CDCl3) δC: 12.28, 18.90, 23.66, 25.54, 26.92, 27.70, 30.30, 31.82, 34.90, 36.62, 37.87, 44.89, 48.12, 52.80, 62.12, 80.10, 80.66, 83.42, 112.72, 115.34, 126.5, 132.24, 138.34, 153.02 ppm. EI-MS m/z: 368.23 Anal. Calcd. for C24H32O3: C, 78.22; H, 8.75; O, 13.02. Found: C, 78.16; H, 8.64.
3-(Tert-Buthyl-Dimethyl-Silanyloxy)-17-(6-Hydroxy-Hex-1-Ynyl)-13-Methyl-7,8,9,11,12, 13,14,15,16,17-Decahydro-6H-Cyclopenta[A]Phenanthrene-17-ol
yielding 66 % of product, m.p. 176-178oC; IR (Vmax, cm-1): 3400, 2198 and 1098; 1H NMR (300 MHz, CDCl3) δH: 0.28 (s. 6H), 0.92 (s, 3H), 1.04 (s, 9H), 1.22-1.50 (m,4H), 1.58-1.60 (m, 4H), 1.70-2.10 (m, 6H), 2.20 (t, 2H, J = 13.47 Hz), 2.24- 2.82 (m, 5H), 3.66 (t, 2H, J = 11.00 Hz), 3.84 (broad, 3H), 6.84-7.28 (m, 3H) ppm. 13C NMR (75.4 Hz, CDCl3) δC: -4.44, 12.28, 18.16, 18.90, 23.66, 25.54, 25.74, 26.92, 27.70, 29.62, 31.82, 34.90, 36.62, 37.87 44.89, 48.12, 52.80, 62.12, 80.10, 80.66, 83.42, 117.10, 119.94, 126.14, 132.64, 137.84, 153.32 ppm. EI-MS m/z: 482.77 Anal. Calcd. for C30H46O3Si: C, 74.64; H, 9.60; O, 9.94 Found: C, 74.56; H, 9.52.
Esterification 3 or 4 With Succinic Acid to form two Dioxaspiro-Steroid-Cyclotridecan Derivatives (5 or 6).
A solution of 5 or 6 (0.5 mmol), succinic acid (100 mg, 0.85 mmol), 1,3-dicyclohexylcarbodiimide (120 mg, 0.58 mmol), p-toluensulfonic acid monohydrate 260 mg (1.36 mmol) in acetonitile:methanol 6 ml (1:2) was stirring for 72 h to room temperature. The reaction mixture was evaporated to dryness under reduced pressure. After, the residue was purified by crystallization from methanol:water:hexane (4:2:1)
4-(((13S)-13-Methyl-2´,5´-Dioxo-6,7,8,9,11,12,13,14,15,16-Decahydro-1´,6´-Dioxaspiro [Cyclopenta[A]Phenanthrene-17,7´-Cyclotridecan]-8´-Yn-3-Yl)Oxy)-4-Oxobutanoic Acid
yielding 48 % of product, m.p. 170-172oC; IR (Vmax, cm-1): 2194, 1750 and 1714; 1H NMR (300 MHz, CDCl3) δH: 0.98 (s, 3H), 1.08 (m, 2H), 1.22-1.44 (m,3H), 1.50 (t, 2H, J = 6.45 Hz), 1.58-2.22 (m, 8H), 2.24 (t, 2H, J = 14.40 Hz), 2.38-2.46 (m, 2H), 2.54- 2.58 (m, 4H), 2.60 (t, 2H, J = 16.00 Hz), 2.68-2.76 (m, 2H), 2.90 (m, 2H), 4.10 (m, 2H), 6.78-7.28 (m, 3H), 8.70 (broad, 1H) ppm. 13C NMR (75.4 Hz, CDCl3) δC: 10.90, 19.20, 23.64, 26.74, 27.70, 28.60, 29.30, 29.70, 29.74, 30.00, 31.72, 32.10, 34.32, 34.84, 38.35, 44.89, 47.82, 52.80, 64.82, 79.10, 80.99, 89.02, 119.50, 121.44, 126.64, 138.14, 139.44, 149.80, 171.30, 172.04, 173.70, 174.12 ppm. EI-MS m/z: 550.25 Anal. Calcd. for C32H36O8: C, 69.80; H, 6.96; O, 23.24. Found: C, 69.74; H, 6.87.
(13S)-3-((Tert-Butyldimethylsilyl)Oxy)-13-Methyl-6,7,8,9, 11,12,13,14,15,16-Decahydro-1´,6´-Dioxaspiro[Cyclopenta [A]Phenanthrene-17,7´-Cyclotridecan]-8´-Yne-2´,5´-Dione
yielding 64 % of product, m.p. 160-162oC; IR (Vmax, cm-1): 2196, 1746 and 1716; 1H NMR (300 MHz, CDCl3) δH: 0.28 (s, 6H), 0.98 (s, 3H), 1.08 (m, 2H), 1.10 (s, 9), 1.22-1.44 (m,3H), 1.50 (t, 2H, J = 1.70 Hz), 1.58-2.22 (m, 8H), 2.24 (t, 2H, J = 1.70 Hz), 2.38-2.46 (m, 2H), 2.54- 2.58 (m, 4H), 2.80-2.82 (m, 2H), 4.10 (t, 2H, J = 2.00 Hz), 6.84-7.28 (m, 3H) ppm. 13C NMR (75.4 Hz, CDCl3) δC: -4.44, 10.90, 18.16, 19.20, 23.66, 25.72, 26.74, 27.70, 28.60, 29.60, 30.00, 31.72, 32.10, 34.32, 34.84, 38.36, 44.89, 47.82, 52.80, 64.82, 79.10, 80.99, 89.02, 117.20, 119.94, 126.14, 133.40, 137.74, 153.42, 172.04, 173.70 ppm. EI-MS m/z: 564.32 Anal. Calcd. for C34H48O5Si: C, 72.30; H, 8.57; O, 14.16; Si, 4.97. Found: C, 72.22; H, 8.48.
Preparation of Diazaspiro[Bicycle[9.4.2]Heptadecane-Steroid-4 Amino Complex (7 or 8)
A solution of 5 or 6 (0.5 mmol), ethylenediamine (60 µl, 0.90 mmol) boric acid (60 mg, 0.97 mmol) in 5 ml of methanol was stirring for 72 h to room temperature. The reaction mixture was evaporated to dryness under reduced pressure. After, the residue was purified by crystallization from methanol:water (4:2)
(11Z,13´S)-13´-Methyl-6´,7´,8´,9´,11´,12´,13´,14´,15´,16´-Decahydro-2,10-Dioxa-12,15-Diazaspiro[Bicycle[9.4.2]Heptadecane-3,17´-[Cyclopenta[A]Phenanthrene]-1(15),11-Dien-4-Yn-3´-Yl 4-Amino-4-Oxobutanoate
yielding 56 % of product, m.p. 186-188oC; IR (Vmax, cm-1): 3324, 2194, 1748 and 1632; 1H NMR (300 MHz, CDCl3) δH: 0.98 (s, 3H), 1.08 (m, 2H), 1.22-1.64 (m, 6H), 1.70 (t, 2H, J = 6.00 Hz), 1.72-1.90 (m, 2H), 1.98 (t, 2H, J = 14.40 Hz), 2.00-2.24 (m, 3H), 2.30 (t, 2H, J = 14.60 Hz), 2.34 (m, 1H), 2.40 (t, 2H, J = 15.42 Hz), 2.68-2.76 (m, 2H), 2.96-3.02 (m, 4H), 3.12 (m, 2H), 4.34 (m, 4H), 5.96 (broad, 2H), 6.70-7.28 (m, 3H) ppm. 13C NMR (75.4 Hz, CDCl3) δC: 11.80, 19.34, 23.34, 24.00, 25.44, 25.50, 27.70, 27.74, 28.00, 29.70, 30.00, 31.78, 33.70, 35.22, 37.38, 44.89, 45.20, 47.45, 50.85, 53.57, 67.82, 80.00, 81.00, 81.62, 118.90, 120.84, 126.64, 138.14, 138.74, 149.40, 164.40, 169.16, 173.70, 177.52 ppm. EI-MS m/z: 573.32 Anal. Calcd. for C34H43N3O5: C, 71.18; H, 7.55; N, 7.32; O, 13.94. Found: C, 71.08; H, 7.48.
(11Z,13´S)-3´-((Tert-Butyldimethylsilyl)Oxy)-13´Methyl-6´,7´,8´,9´,11´,12´,13´,14´,15´, 16´-Decahydro-2,10-Dioxa-12,15-Diazaspiro[Bicycle[9.4.2]Heptadecane-3,17´-[Cyclo- Penta [A]Phenanthrene]-1(15),11-Dien-4-Yne
yielding 55 % of product, m.p. 134-136oC; IR (Vmax, cm-1): 3398 and 2192; 1H NMR (300 MHz, CDCl3) δH: 0.28 (s, 6H), 0.98 (s, 3H), 1.04 (s, 9H), 1.08 (m, 2H), 1.22-1.62 (m, 6H), 1.70 (t, 2H, J = 6.45 Hz), 1.72-1.92 (m, 3H), 1.98 (t, 2H, J = 14.40 Hz), 2.00-2.82 (m, 6H), 2.96-3.00 (m, 4H), 3.12 (m, 2H), 4.34 (m, 4H), 6.84-7.28 (m, 3H) ppm. 13C NMR (75.4 Hz, CDCl3) δC: -4.44, 11.80, 18.16, 19.36, 23.32, 24.00, 25.42, 25.50, 25.68, 27.70, 27.74, 28.00, 29.60, 33.70, 36.22, 37.40, 44.89, 45.18, 47.46, 50.88, 53.57, 67.82, 79.00, 81.00, 81.62, 117.20, 119.94, 126.14, 132.70, 137.74, 153.42, 164.42, 173.70 ppm. EI-MS m/z: 588.37 Anal. Calcd. for C36H52N2O3Si: C, 73.42; H, 8.90; N, 4.76; O, 8.15; Si, 4.77. Found: C, 73.34; H, 8.82.
Removal of Silyl Fragment of 8 Via Hydrofluoric Acid to form
A solution of 8 (200 mg 0.34 mmol), in 5 ml hydrofluoric acid was stirring for 12 h to room temperature. The reaction mixture was evaporated to dryness under reduced pressure. After, the residue was purified by crystallization from methanol:water (3:2)
(11Z,13´S)-13´-Methyl-6´,7´,8´,9´,11´,12´,13´,14´,15´,16´-Decahydro-2,10-Dioxa-12,15-Diazaspiro[Bicycle[9.4.2] Heptadecane-3,17´-[Cyclopenta[A]Phenanthrene]-1(15),11-Dien-4-Yn-3´-ol
yielding 76 % of product, m.p. 248-250oC; IR (Vmax, cm-1): 3398, 3322, 2192; 1H NMR (300 MHz, CDCl3) δH: 0.98 (s, 3H), 1.10 (m, 2H), 1.22-1.62 (m, 6H), 1.70 (t, 2H, J = 6.45 Hz), 1.72-1.92 (m, 3H), 1.98 (t, 2H, J = 14.40 Hz), 2.00-2.76 (m, 6H), 2.96-3.00 (m, 4H), 3.12 (m, 2H), 4.34 (m, 4H), 6.74-7.28 (m, 3H) ppm 8.96 (broad, 1H). 13C NMR (75.4 Hz, CDCl3) δC: 11.80, 19.36, 23.32, 24.00, 25.42, 25.50, 27.70, 27.74, 28.00, 30.26, 33.70, 35.22, 37.40, 44.89, 45.18, 47.46, 50.88, 53.57, 67.82, 79.00, 81.00, 81.62, 113.32, 115.64, 126.94, 132.30, 138.44, 153.22, 164.42, 173.70 ppm. EI-MS m/z: 474.28 Anal. Calcd. for C30H38N2O3: C, 75.92; H, 8.07; N, 5.90; O, 10.11. Found: C, 75.84; H, 8.00.
Preparation of Diazaspiro[Bicycle[9.4.2]Heptadecane-Steroid-4-Oxobutanoic Acid (10) Via Esterification of
A solution of 9 (200 mg 0.42 mmol), succinic acid (100 mg, 0.85 mmol), 1,3-dicyclohexylcarbodiimide (120 mg, 0.58 mmol), p-toluensulfonic acid monohydrate 260 mg (1.36 mmol) in acetonitile:methanol 6 ml (1:2) was stirring for 72 h to room temperature. The reaction mixture was evaporated to dryness under reduced pressure. After, the residue was purified by crystallization from methanol:water:hexane (3:1)
4-(((11Z,13´S)-13´-Methyl-6´,7´,8´,9´,11´,12´,13´,14´,15´,16´-Decahydro-2,10-Dioxa-12, 15-Diazaspiro[Bicycle[9.4.2]Heptadecane-3,17´-[Cyclopenta[A]Phenanthrene]-1(15),11-Dien-4-Yn-3´-Yl)Oxy)-4-Oxobutanoic Acid
yielding 76 % of product, m.p. 180-182oC; IR (Vmax, cm-1): 3324, 2192, 1748 and 1712; 1H NMR (300 MHz, CDCl3) δH: 0.98 (s, 3H), 1.10 (m, 2H), 1.22-1.62 (m, 6H), 1.70 (t, 2H, J = 6.45 Hz), 1.72-1.92 (m, 3H), 1.98 (t, 2H, J = 14.40 Hz), 2.00-2.34 (m, 4H), 2.60 (m, 2H), 2.64-2.74 (m, 2H), 2.90 (m, 2H), 2.96-3.00 (m, 4H), 3.12 (m, 2H), 4.34 (m, 4H), 6.74-7.28 (m, 3H) ppm 8.66 (broad, 1H). 13C NMR (75.4 Hz, CDCl3) δC: 11.80, 19.36, 23.32, 24.00, 25.42, 25.50, 27.70, 27.74, 28.00, 29.32, 29.70, 29.74, 33.70, 35.22), 37.40, 44.89, 45.18, 47.46, 50.88, 53.57, 67.82, 79.00, 81.00, 81.62, 119.32, 121.44, 126.64, 138.14, 138.76, 149.72, 164.42, 171.28, 173.70, 174.12 ppm. EI-MS m/z: 574.30 Anal. Calcd. for C34H42N2O6: C, 71.06; H, 7.37; N, 4.87; O, 16.70. Found: C, 71.00; H, 7.24.
Amidation of 10 With Ethylenediamine to form Diazaspiro[Bicycle[9.4.2] Heptadecane-Steroid-4-Aminobutanoate
A solution of 10 (200 mg 0.35 mmol), ethylenediamine (80 µl, 0.74 mmol) boric acid (40 mg, 0.65 mmol) in 10 mL of methanol was stirring for 24 h at room temperature. The reaction mixture was evaporated to dryness under reduced pressure. After, the residue was purified by crystallization from methanol:water (3:2), yielding 67 % of product. Similar 1H NMR and 13C NMR data were obtained compared with the reaction of 5 with ethylenediamine ethylenediamine to form 7.
Reduction of Amide Group of 7 to form
A solution of 7 (200 mg 0.34 mmol), NaBH3CN (30 mg, 0.48 mmol) in 10 ml of dioxane:water (3:2) was stirring for 72 h to room temperature. The reaction mixture was evaporated to dryness under reduced pressure. After, the residue was purified by crystallization from methanol:water (4:2).
(11Z,13´S)-13´-Methyl-6´,7´,8´,9´,11´,12´,13´,14´,15´,16´-Decahydro-2,10-Dioxa-12,15-Diazaspiro[Bicyclo[9.4.2]Heptadecane-3,17´-Cyclopenta[A]Phenantherene]1(15),11-Dien-4-Yn-3´-Yl 4-Aminobutanoate
yielding 60 % of product, m.p. 230-232oC; IR (Vmax, cm-1): 3322, 2194 and 1748; 1H NMR (300 MHz, CDCl3) δH: 0.98 (s, 3H), 1.08 (m, 2H), 1.22-1.54 (m, 4H), 1.56 (t, 2H, J = 6.72 Hz), 1.60-1.63 (m, 2H), 1.70 (t, 2H, J = 6.00 Hz), 1.72-1.76 (m, 2H), 1.84 (broad, 2H), 1.90 (m, 1H), 1.98 (m, 2H), 2.00-2.34 (m, 4H), 2.36 (t, 2H, J = 15.42 Hz), 2.66-2.77 (m, 2H), 2.80 (m, 2H), 2.96-3.02 (m, 4H), 3.12 (m, 2H), 4.34 (m, 4H), 6.73-7.28 (m, 3H) ppm. 13C NMR (75.4 Hz, CDCl3) δC: 11.80, 19.34, 23.34, 24.00, 25.44, 25.50, 26.32, 27.70, 27.74, 28.00, 29.70, 30.80, 33.70, 35.22, 37.38, 40.64, 44.89, 45.20, 47.45, 50.85, 53.57, 67.82, 79.00, 81.00, 81.62, 119.30, 121.24, 126.64, 138.14, 138.74, 148.80, 164.40, 168.64, 173.70 ppm. EI-MS m/z: 559.34 Anal. Calcd. for C34H45N3O4: C, 72.96; H, 8.10; N, 7.51; O, 11.43. Found: C, 72.88; H, 7.42.
Synthesis of Dioxa-Diazaspiro[Bicyclo[9.4.2]Hepta- Decane-Steroid-Dienyne (12) Via Pyrrolization of
A solution of 11 (200 mg 0.38 mmol), ethylenediamine (80 µl, 0.74 mmol) boric acid (40 mg, 0.65 mmol) in 10 ml of methanol was stirring for 72 h to room temperature. The reaction mixture was evaporated to dryness under reduced pressure. After, the residue was purified by crystallization from methanol:water (4:1)
(11Z,13´S)-3´-((3,4-Dihydro-2H-Pyrrol-5-Yl)Oxy)-13´-Methyl-6´,7´,8´,9´,11´,12´,13´,14´, 15´,16´-Decahydro-2,10-Dioxa-12,15-Diazaspiro[Bicyclo[9.4.2]Heptadecane-3,17´-Cyclopenta[A]Phenantherene]1(15),11-Dien-4-Yne
yielding 52 % of product, m.p. 158-160oC; IR (Vmax, cm-1): 3324 and 2192; 1H NMR (300 MHz, CDCl3) δH: 0.98 (s, 3H), 1.08 (m, 2H), 1.22-1.62 (m, 6H), 1.70 (t, 2H, J = 6.00 Hz), 1.72-1.90 (m, 2H), 1.98 (m, 2H), 2.00 (m, 1H), 2.10-2.18 (m, 2H), 2.24-2.76 (m,4H), 2.76 (m, 1H), 2.77 (m, 1H), 2.80 (m, 1H), 2.84 (m, 1H), 2.96-3.02 (m, 4H), 3.12 (m, 2H), 4.14 -4.20 (m, 2H), 4.34 (m, 4H), 6.90-7.28 (m, 3H) ppm. 13C NMR (75.4 Hz, CDCl3) δC: 15.82, 19.34, 20.50, 23.34, 23.76, 25.44, 25.50, 27.58, 27.90, 28.00, 29.40, 29.80, 33.70, 33.74, 38.78, 43.34, 44.11, 47.45, 50.85, 52.22, 56.90, 67.82, 79.00, 81.00, 81.62, 115.76, 118.80, 127.84, 137.00, 139.84, 155.50, 164.40, 167.24, 173.70 ppm. EI-MS m/z: 541.33 Anal. Calcd. for C34H43N3O3: C, 75.38; H, 8.00; N, 7.76; O, 8.86. Found: C, 75.26; H, 7.92.
Results and Disscusion
There are reports which indicate the preparation of diverse bicycle derivatives; nevertheless, expensive reagents and special conditions are required. Therefore, in this study a dioxa-diazaspiro[bicyclo[9.4.2]heptadecane-steroid-dienyne derivative from estrone and OTBS-estrone was synthetized using several strategies.
Propargylic-Alcohols Derivatives Via Reaction of Terminal Alkynes With Ketone Group (3 or 4)
There are several reports which showed the preparation of some propargylic-alcohols using different methods and reagents such as disulfide-oxazolidine10, Ti(O-i-Pr)4-BINOL complex11, chiral diamine-coordinated tin(II) triflate12, P(PhCH2NCH2CH2)3N13 and others; however some of these reagents are difficult to handle require and special conditions. Therefore, in this work the estrone was reacted with 5-Hexyn-1-ol in basic medium (Scheme 2). The mechanism of reaction involves a mechanism via SN2. The 1H NMR spectrum of 3 showed several signals at 0.92 ppm for methyl group; at 1.22-1.50, 1.70-2.10, 2.24-2.76 and 6.48-7.10 ppm for steroid moiety; at 1.58-1.60, 2.20, and 3.66 ppm for methylene groups involved in the arm bound to both D-ring of steroid and alkyne group; at 5.54 ppm for hydroxyl groups. The 13C NMR spectra displays chemical shifts at 12.128 ppm for methyl group; at 18.90, 25.54, 31.82 and 62.12 for methylene groups involved in the arm bound to both D-ring of steroid and alkyne group; at 23.66, 26.92-30.30, 34.90-52.80, 80.10 and 112.72-153.02 ppm for steroid moiety; at 80.66-83.42 ppm for carbons of alkyne group. Finally, the presence of compound 3 was confirmed with mass spectrum which showed a molecular ion at 368.23.
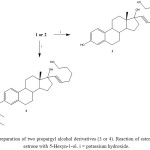 |
Scheme 2: Preparation of two propargyl alcohol derivatives (3 or 4). Reaction of estrone or OTBS-estrone with 5-Hexyn-1-ol. i = potassium hydroxide. Click here to View scheme |
On the other hand the 1H NMR spectrum of 4 showed several signals at 0.28 and 1.04 ppm for ter-buthyldimethylsylane fragment; at 0.92 ppm for methyl group; at 1.22-1.50, 1.70-2.10, 2.24-2.82 and 6.84-7.28 ppm for steroid moiety; at 1.58-1.60, 2.20, and 3.66 ppm for methylene groups involved in the arm bound to both D-ring of steroid and alkyne group; at 3.84 ppm for hydroxyl group. The 13C NMR spectra displays chemical shifts at -4.44, 18.16 and 25.70 ppm for carbos of ter-buthyldimethylsylane fragment; at 12.128 ppm for methyl group; at 18.90, 25.54, 31.82 and 62.12 ppm for methylene groups involved in the arm bound to both D-ring of steroid and alkyne group; at 23.66, 26.92-29.62, 34.90-52.80, 80.10 and 117.10-153.32 ppm for steroid moiety; at 80.66-83.42 for carbons of alkyne group. Additionally, the presence of compound 4 was confirmed with mass spectrum which showed a molecular ion at 482.77.
Esterification 3 or 4 With Succinic Acid to form two Dioxaspiro-Steroid-Cyclotridecan Derivatives (5 or 6).
There are diverse reagents used as catalyst to preparing of ester derivatives14,15; however, most of the conventional methods are of limited use for some compounds. Therefore, in this study the method reported by Erlanger and coworkers16 for esterification of other compounds was used. Thus, compounds 5 or 6 were prepared by the reaction of 3 or 4 with succinic acid using 1,3-dicyclohexylcarbodiimide (DCC) as coupling reagent. It is important to mention that when DCC is used alone as a condensing agent in ester synthesis, the yield of esters is often unsatisfactory due to formation of an N-acylurea by-product. Some reports showed that addition of a catalytic amount of a strong acid to the esterification reaction in the presence of DCC considerably increases the yield of esters and decreases the formation of N-acylurea3. Therefore, p-toluenesulfonic acid was used to increase the yield of 5 or 6 in the esterification of 3 or 4 with succinic acid in the presence of DCC (Scheme 3). The 1H NMR spectrum of 5 showed several signals at 0.98 ppm for methyl group; at 1,08, 1.50, 2.24 and 4.10 ppm for methylene groups bound to both alkyne and ester groups; at 2.54-2.58 ppm for methylene groups bound to both ester groups; at 1.22-1.44, 1.58-2.22, 2.38-2.46, 2.68-2.76 and 6.78-7.28 ppm for steroid moiety; at 2.60-2.90 ppm for methylene groups bound to ester an carboxyl groups; at 8.70 ppm for carboxyl group. The 13C NMR spectra displays chemical shifts at 10.90 for methyl group; at 19.20, 28.60, 32.10 and 64.82 ppm for methylene bound to both ester and alkyne groups; at 30.00-31.72 ppm for methylene groups bound to both ester groups; at 23.64-28.70, 29.70, 34.32-52.80, 80.99 and 119.50-149.80 ppm for steroid moiety; at 29.30 and 29.74 ppm for methylene groups bound to both ester and carboxyl groups; at 79.10 and 89.02 ppm for alkyne group; at 171.30-173.70 ppm for carbons of ester groups; at 174.12 ppm for carboxyl group. Finally, the presence of compound 5 was confirmed with mass spectrum which showed a molecular ion at 550.25.
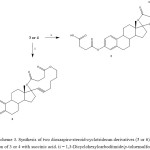 |
Scheme 3: Synthesis of two dioxaspiro-steroid-cyclotridecan derivatives (5 or 6). Reaction of 3 or 4 with succinic acid. ii = 1,3-Dicyclohexylcarbodiimide/p-toluensulfonic acid. Click here to View scheme |
On the other hand, the 1H NMR spectrum of 6 showed several signals at 0.28 and 1.10 ppm for tert-buthyldimethylsylane fragment; at 0.98 ppm for methyl group; at 1,08, 1.50, 2.24 and 4.10 ppm for methylene groups bound to both alkyne and ester groups; at 2.54-2.58 ppm for methylene groups bound to both ester groups; at 1.22-1.44, 1.58-2.22, 2.38-2.46, 2.80-2.82 and 6.84-7.28 ppm for steroid moiety; at 2.54-2.58 ppm for methylene groups bound to ester an carboxyl groups. The 13C NMR spectra displays chemical shifts at -4.44, 18.16 and 25.72 ppm for tert-buthyldimethylsylane fragment; at 10.90 for methyl group; at 19.20, 28.60, 32.10 and 64.82 ppm for methylene bound to both ester and alkyne groups; at 30.00-31.72 ppm for methylene groups bound to both ester groups; at 23.66, 26.74-27.70, 29.60, 34.32-52.80, 80.99 and 117.20-153.42 ppm for steroid moiety; at 79.10 and 89.02 ppm for alkyne group; at 172.04-173.70 ppm for carbons of ester groups. In addition, the presence of compound 6 was confirmed with mass spectrum which showed a molecular ion at 564.32.
Preparation of Diazaspiro[Bicycle[9.4.2]Heptadecane-Steroid-4 Amino Complex (7 or 8)
There are several methods for preparation of ether derivatives which involve the use of different reagents such hexyl bromide/sodium cyanide17, m-chloroperoxybenzoic acid18, hydrazonyl chloride19, N,N-dimethylbarbituric acid20 and others. In this study, the compounds 7 or 8 were prepared via formation of imino group from 5 or 6 with ethylenediamine in presence of boric acid (Scheme 4). It is also noteworthy, that dditionally compound 7 involves the formation of an amide group participating in the arm attached to A-ring of steroid. The 1H NMR spectrum of 7 showed several signals at 0.98 ppm for methyl group; at 1,08, 1.70, 1.98 and 3.12 ppm for methylene groups bound to both alkyne and ester groups; at 1.22-1.64, 1.72-1.90, 2.00-2.24, 2.34, 2.68-2.76 and 6.70-7.28 ppm for steroid moiety; at 2.30-2.40 ppm for methylene groups bound to ester an carboxyl groups; at 2.96-3.02 and 4.34 ppm for methylene groups bound to imino groups; at 5.96 ppm for carboxyl group. The 13C NMR spectra displays chemical shifts at 11.80 for methyl group; at 19.34, 25.44-25.50 and 67.82 ppm for methylene bound to both ester and alkyne groups; at 23.34, 28.00, 47.45-50.85 ppm for methylene groups bound to imino groups; at 24.00, 27.70-27.74, 29.70, 33.70-45.20, 53.57, 80.00 and 118.90-149.40 ppm for steroid moiety; at 30.00-31.78 ppm for methylene groups bound to both ester and carboxyl groups; at 81.00 and 86.62 ppm for alkyne group; at 164.40 and 173.70 ppm for imino groups; at 169.16 ppm for ester group; at 177.52 ppm for carboxyl group. Finally, the presence of compound 7 was confirmed with mass spectrum which showed a molecular ion at 573.32.
![Scheme 4: Preparation of diazaspiro[bicycle[9.4.2]heptadecane-steroid-4 amino complex (7 or 8). Reaction of 5 or 6 with ethylenediamine to form 7 or 8. i = boric acid.](http://www.orientjchem.org/wp-content/uploads/2017/06/Vol33No3_Desi_Ram_sch4-150x150.jpg) |
Scheme 4: Preparation of diazaspiro[bicycle[9.4.2]heptadecane-steroid-4 amino complex (7 or 8). Reaction of 5 or 6 with ethylenediamine to form 7 or 8. i = boric acid. Click here to View scheme |
On the other hand, the 1H NMR spectrum of 8 showed several signals at 0.28 and 1.04 ppm for tert-buthyldimethylsylane fragment; at 0.98 ppm for methyl group; at 1.08, 1.70, 1.98 and 3.12 ppm for methylene groups bound to both alkyne and ester groups; at 1.22-1.62, 1.72-1.92, 2.00- 2.82 and 6.84-7.28 ppm for steroid moiety; at 2.96-3.00 and 4.34 ppm for methylene groups bound to imino groups. The 13C NMR spectra displays chemical shifts at -4.44, 18.16 and 25.68 ppm for tert-buthyldimethylsylane fragment; at 11.80 ppm for methyl group; at 19.36, 28.42-25.50 and 67.82 ppm for methylene bound to both ester and alkyne groups; at 23.34, 28.00, 47.45-50.85 ppm for methylene groups bound to imino groups; at 24.00, 27.70-27.740, 29.60-45.18, 53.57, 79.00 and 117.20-153.42 ppm for steroid moiety; at 81.00-81.62 ppm for alkyne group; at 164.42-173.70 ppm for imino groups. In addition, the presence of compound 8 was confirmed with mass spectrum which showed a molecular ion at 588.37.
Removal of Silyl Fragment of 8 Via Hydrofluoric Acid to form
There are several reagent for removal of silyl protecting groups from hydroxyl such as ammonium fluoride21 [20], tris(dimethylamino)sulfonium/difluorotrimethylsilicate22, hydrofluoric acid23 and others. In this study, hydrofluoric acid was used to removal of silyl-protecting group from hydroxyl of the compound 8 to form 9 (Scheme 5). The 1H NMR spectrum of 9 showed several signals at 0.98 ppm for methyl group; at 1.10, 1.70, 1.98 and 3.12 ppm for methylene groups bound to both alkyne and ester groups; at 1.22-1.62, 1.72-1.92, 2.00- 2.76and 6.74-7.28 ppm for steroid moiety; at 2.96-3.00 and 4.34 ppm for methylene groups bound to imino groups at 8.96 for hydroxyl group. The 13C NMR spectra displays chemical shifts at 11.80 ppm for methyl group; at 19.36, 25.42-25.50 and 67.82 ppm for methylene bound to both ester and alkyne groups; at 23.34, 28.00, 47.45-50.85 ppm for methylene groups bound to imino groups; 24.00, 27.70-27.74, 30.26-45.18, 53.57, 79.00 and 113.32-153.22 ppm for steroid moiety; at 81.00-81.62 ppm for alkyne group; at 164.42-173.70 ppm for imino groups. In addition, the presence of compound 9 was confirmed with mass spectrum which showed a molecular ion at 474.28.
![Scheme 5: Synthesis of diazaspiro[bicyclo[9.4.2]heptadecane-steroid-4-aminobutanoate (7). Reaction of 8 with hydrofluoric acid to form diazaspiro[bicycle[9.4.2]heptadecane-3steroid-dien-yn-3´-ol (9). Then 9 was reacted with succinic acid (v) to form the diazaspiro[bicycle[9.4.2]heptadecane-steroid-4-oxobutanoic acid (10). Finally, 7 was prepared by the reaction of 10 with ethylenediamine (vi).](http://www.orientjchem.org/wp-content/uploads/2017/06/Vol33No3_Desi_Ram_sch5-150x150.jpg) |
Scheme 5: Synthesis of diazaspiro[bicyclo[9.4.2]heptadecane-steroid-4-aminobutanoate (7). Reaction of 8 with hydrofluoric acid to form diazaspiro[bicycle[9.4.2]heptadecane-3steroid-dien-yn-3´-ol (9). Then 9 was reacted with succinic acid (v) to form the diazaspiro[bicycle[9.4.2]heptadecane-steroid-4-oxobutanoic acid (10). Finally, 7 was prepared by the reaction of 10 with ethylenediamine (vi). Click here to View scheme |
Preparation of Diazaspiro[Bicycle[9.4.2]Heptadecane-Steroid-4-Oxobutanoic Acid (10) Via Esterification of
The compound 10 was prepared by the reaction of 9 with succinic acid using 1,3-dicyclohexylcarbodiimide in presence of p-toluenesulfonic acid (Scheme 5). The 1H NMR spectrum of 10 showed several signals at 0.98 ppm for methyl group; at 1.10, 1.70, 1.98 and 3.12 ppm for methylene groups bound to both alkyne and ester groups; at 1.22-1.62, 1.72-1.92, 2.00-2.34 and 6.74-7.28 ppm for steroid moiety; at 2.60, 2.90 ppm for methylene groups bound to both ester and carboxyl groups; at 2.96-3.00 and 4.34 ppm for methylene groups bound to imino groups at 8.96 ppm for hydroxyl group; at 8.66 ppm for carboxyl group. The 13C NMR spectra displays chemical shifts at 11.80 ppm for methyl group; at 19.36, 25.42-25.50 and 67.82 ppm for methylene bound to both ester and alkyne groups; at 23.30, 28.00, 47.46-50.88 ppm for methylene groups bound to imino groups; 24.00, 27.70-27.74, 29.70, 33.70-45.18, 53.57, 79.00 and 119.32-149.72 ppm for steroid moiety; at 81.00-81.62 ppm for alkyne group; at 164.42-173.70 ppm for imino groups; at 171.28 ppm for ester group at 174.12 ppm for carboxyl group. In addition, the presence of compound 10 was confirmed with mass spectrum which showed a molecular ion at 574.30.
Amidation of 10 With Ethylenediamine to form Diazaspiro[Bicyclo[9.4.2]Heptadecane-Steroid-4-Aminobutanoate
Many procedures for the formation of amide groups are known in the literature, the most widely practiced method employs carboxylic acid chlorides as the electrophiles which react with the amine in the presence of an acid scavenger24. Despite its wide scope, this protocol suffers from several drawbacks; most notable are the limited stability of many acid chlorides and the need for hazardous reagents for their preparation (thionyl chloride)25. In this work two different methods for amide formation were employed, in the first one the technique reported by Pingwah26 for boric acid catalyzed amidation of carboxylic acids and amine was used. Therefore, boric acid was used as catalyst in the reaction of 10 with ethylenediamine to form the compounds 7 (Scheme 5). Here, is important to mention that with this method, the yield of 7 was higher compared to the reaction of 5 with ethylenediamine.
Reduction of Amide Group of 7 to form
Several reagents have been used for reduction of amides to form amino groups such as Fe3(CO)1227, LiAlH28, borohydride derivative29 and others. In this study, 7 was reacted with NaBH3CN to form 11. The 1H NMR spectrum of 11 showed several signals at 0.98 ppm for methyl group; at 1,08, 1.70, 1.98 and 3.12 ppm for methylene groups bound to both alkyne and ester groups; at 1.22-1.54, 1.60-1.63, 1.72-1.76, 1.90, 2.00-2.34, 2.66-2.77 and 6.73-7.28 ppm for steroid moiety; at 1.56, 2.36 and 2.80 ppm for methylene groups bound to ester and amino groups; at 1.84 for amino group; at 2.96-3.02 and 4.34 ppm for methylene groups bound to imino groups. The 13C NMR spectra displays chemical shifts at 11.80 for methyl group; at 19.34, 25.44-25.50 and67.82 ppm for methylene bound to both ester and alkyne groups; at 23.34, 28.00, 47.45-50.85 ppm for methylene groups bound to imino groups; at 24.00, 27.70-27.74, 29.70, 33.70-37.38, 44.89-45.20, 53.57, 79.00 and 119.30-148.80 ppm for steroid moiety; at 26.32, 30.80 and 40.64 ppm for methylene groups bound to both ester and amino groups; at 81.00 and 86.62 ppm for alkyne group; at 164.40 and 173.70 ppm for imino groups; at 168.64 ppm for ester group. Finally, the presence of compound 11 was confirmed with mass spectrum which showed a molecular ion at 559.34.
Synthesis of Dioxa-Diazaspiro[Bicyclo[9.4.2]Hepta- Decane-Steroid-Dienyne (12) Via Pyrrolization of
There are different methods for the preparation of pyrrole derivatives30-33; nevertheless, some protocols that require hazardous reagents as well as different experimental conditions for the preparation of these compounds. In this study 11 was reacted with ethylenediamine in presence of boric acid to form an imino group and consequently bring formation of the pyrrole ring involved in the compound 12 (Scheme 6). The 1H NMR spectrum of 12 showed several signals at 0.98 ppm for methyl group; at 1.08, 1.70, 1.98 and 3.12 ppm for methylene groups bound to both alkyne and ester groups; at 1.22-1.62, 1.72-1.90, 2.00, 2.24-2.76, 2.80 and 6.90-7.28 ppm for steroid moiety; at 2.10-2.18, 2.77, 2.84 and 4.14-4.20 ppm for pyrrole ring; at 2.96-3.02 ppm for methylene groups bound to imino groups. The 13C NMR spectra displays chemical shifts at 15.82 for methyl group; at 19.34, 25.44-25.50 and 67.82 ppm for methylene bound to both ester and alkyne groups; at 23.34, 28.00, 47.45-50.85 ppm for methylene groups bound to imino groups; at 23.76, 25.58-27.90, 33.70-44.11, 52.22, 79.00 and 115.76-155.50 ppm for steroid moiety; at 26.32, 30.80 and 40.64 ppm for methylene groups bound to both ester and amino groups; at 81.00 and 86.62 ppm for alkyne group; at 164.40 and 173.70 ppm for imino groups. Finally, the presence of compound 12 was confirmed with mass spectrum which showed a molecular ion at 541.33.
![Scheme 6: Synthesis of dioxa-diazaspiro[bicyclo[9.4.2]heptadecane-steroid-dienyne (12). Reaction of 7 with NaBH3CN to form the compound diazaspiro[bicyclo[9.4.2] heptadecane-steroid 4-aminobutanoate derivative (11). Following, 11 was reacted with ethylenediamine/boric acid to form 12.](http://www.orientjchem.org/wp-content/uploads/2017/06/Vol33No3_Desi_Ram_sch6-150x150.jpg) |
Scheme 6: Synthesis of dioxa-diazaspiro[bicyclo[9.4.2]heptadecane-steroid-dienyne (12). Reaction of 7 with NaBH3CN to form the compound diazaspiro[bicyclo[9.4.2] heptadecane-steroid 4-aminobutanoate derivative (11). Following, 11 was reacted with ethylenediamine/boric acid to form 12. Click here to View scheme |
Conflict of Interest
We declare that this manuscript does not have any conflict of financial interests (political, personal, religious, ideological, academic, intellectual, commercial or otherwise) for its publication.
Conclusions
In this study is reported a straightforward route for synthesis of a dioxa-diazaspiro [bicyclo[9.4.2]heptadecane-steroid-dienyne derivative using some strategies. The proposed methods offer some advantages such as simple procedure, low cost, and ease of workup.
References
- Kaszynski, P.; Michl, J. J. Org. Chem. 1988, 53, 4593-4594.
CrossRef - Nicolaou, K.; Pfefferkorn, J.; Guo, C.; Kim, S.; Kessabi, J. Org. Lett. 1999, 1, 807-810.
CrossRef - Hernandez, A.; Rapoport, M. J. Org. Chem. 1995, 60, 2683-2691.
CrossRef - Subba, B.; Venkateswarlu, A.; Borkar, P.; Yadav, J.; Kanakaraju, M.; Kunwar, A.; Sridhar, A. J. Org. Chem. 2013, 78, 6303-6308.
CrossRef - Subba, B.; Borkar, P.; Chakravarthy, P.; Yadav, J.; Gree, R. Tetrahedron Lett. 2010, 51, 3412-3416.
CrossRef - Rueping, M..; Kuenkel, A.; Fröhlich, R. Chem. Eur. J. 2010, 16, 4173-4176.
CrossRef - Rigby, J.; Sage, J. J. Org. Chem. 1983, 48, 3591-3592.
CrossRef - Harding, K.; Trotter, J.; May, L. J. Org. Chem. 1977, 42, 2715-2719.
CrossRef - Figueroa-Valverde, L.; Díaz-Cedillo, F.; Rosas-Nexticapa, M.; Hau-Heredia, L.; García-Cervera, E.; Pool-Gómez, E.; Sarabia-Alcocer, B. J. Chem. In line, 2014, Article ID 460968, http://dx.doi.org/10.1155/2014/460968
CrossRef - Braga, L.; Appelt, H.; Silveira, C.; Wessjohannb, L.; Schneider, P. Tetrahedron. 2002, 58, 10413-10416.
CrossRef - Marshall, J.; Bourbeau, M. Org. Lett. 2003 5, 3197-3199.
CrossRef - Mukaiyama, T.; Furuya, M.; Ohtsubo, A.; Kobayashi, S. Chem. Lett. 1991, 20, 989-992.
CrossRef - Wadhwa, K.; Chintareddy, V.; Verkade, J. J. Org. Chem. 2009, 74, 6681-6690.
CrossRef - Nahar, L.; Turner, A. Steroids. 2003, 68, 1157-1161.
CrossRef - Medvedeva, M.; Safronova, A.; Sarapulova, G. Arkivoc. 2001, ix, 143-149.
- Erlanger, B.; Borek, F.; Beiser, S.; Lieberman, S. J. Biol. Chem. 1959, 234, 1090-1094.
- Benfenati, E.; Fanelli, R.; Bosone, E.; Biffi, C.; Caponi, R.; Cianetti, M.; Farina, P. Drug Metab. Dispos. 1991, 19, 913-916.
- Yates, P.; Hoare, J. Canadian J. Chem. 1983, 61, 1397-1404.
CrossRef - Farag, A.; Korany, E. J. Heteroc. Chem. 2008, 45 279-283.
- Islam, M.; Barakat, A.; Al-Majid, A.; Ghabbour, H.; Fun, H.; Rafiq, M. Arabian J. Chem. 2015, online, doi:10.1016/j.arabjc.2015.03.007
CrossRef - Zhang, W.; Robins, M. Tetrahedron Lett. 1992, 33, 1177-1180.
CrossRef - Scheidt, K.; Chen, H.; Follows, B.; Chemler, S.; Coffey, D.; Roush, W. J. Org. Chem. 1998, 63, 6436-6437.
CrossRef - Newton, R.; Reynolds, D.; Finch, M.; Kelly, D.; Roberts, S. 1979, 20, 3981-3982.
- Armstrong, S.; Bertozzi, J. Org. Lett. 2000, 2, 2141-2143.
- Taddei, M. Org. Lett. 1999, 1, 1355-1357.
CrossRef - Figueroa-Valverde, L.; Díaz-Cedillo, F.; Ceballos-Reyes, G. J. Mex. Chem. Soc. 2006, 50, 42-45.
- Zhou, S.; Junge, K.; Addis, D.; Das, S.; Beller, M. Agew. Chem. 2009, 121, 9671-9674.
CrossRef - Douat, C.; Heitz, A.; Martinez, J.; Fehrentz, J. Tetrahedron Lett. 2000, 41, 37-40.
CrossRef - Satoh, T.; Suzuki, S. Tetrahedron Lett. 1969, 4555-4558.
CrossRef - Schley, N.; Dobereiner, E.; Crabtree, H. Organometallics. 2011, 30, 4174-4179.
CrossRef - Zhang, M.; Neumann, H.; Beller, M. Angew. Chem. 2013, 125, 625-6.29.
CrossRef - Kanchithalaivan, S.; Kumar, R.; Perumal, S. Steroids. 2013, 78, 409-417.]
CrossRef - Crawley, M.; Goljer, I.; Jenkins, D.; Mehlmann, J.; Nogle, L.; Dooley, R.; Mahaney, P. Org. Lett. 2006, 8, 5837-5840.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









