Kinetics and Thermodynamics of Oxidation of Some Meta-Substituted Anilinesby Tetrabutylammoniumbromochromatein Aqueous Acetic Acid Medium
Shaik Jabir1, Basim H. Asghar2 and S. Sheik Mansoor3
1Research and Development Centre, Bharathiar University, Coimbatore – 641 046, Tamil Nadu, India.
2Department of Chemistry, Faculty of Applied Sciences, Umm Al-Qura University, P.O. Box: 9569, Makkah, Saudi Arabia.
3Department of Chemistry, C. Abdul Hakeem College (Autonomous), Melvisharam - 632 509, Tamil Nadu, India.
Corresponding Author E-mail: smansoors2000@yahoo.co.in
DOI : http://dx.doi.org/10.13005/ojc/330134
The tetrabutylammoniumbromochromate(TBABC) oxidation of anilines, in anaqueous acetic acid mediumin the presence of perchloric acid is described. The reaction is first order with respect to aniline, TBABC and acid. The reaction rate has been determined at different temperatures and activation parameters calculated. The TBABC oxidation of meta-substituted anilines obeys Hammett relationships
KEYWORDS:tetrabutylammoniumbromochromate;aniline; thermodynamic parameters; kinetics
Download this article as:| Copy the following to cite this article: Jabir S, Asghar B. H, Mansoor S. S. Kinetics and Thermodynamics of Oxidation of Some Meta-Substituted Anilinesby Tetrabutylammoniumbromochromatein Aqueous Acetic Acid Medium. Orient J Chem 2017;33(1). |
| Copy the following to cite this URL: Jabir S, Asghar B. H, Mansoor S. S. Kinetics and Thermodynamics of Oxidation of Some Meta-Substituted Anilinesby Tetrabutylammoniumbromochromatein Aqueous Acetic Acid Medium. Orient J Chem 2017;33(1). Available from: http://www.orientjchem.org/?p=28381 |
Introduction
Aniline is known to be carcinogenic and also reacts easily in the blood to convert haemoglobin into methaemoglobin, preventing oxygen uptake. Aniline and its derivatives are used as intermediates for the manufacturing of various organic compounds such as colorants, agrochemicals and pharmaceutical agents.Therefore, a serious effect on human health over a long period of time is possible, even if aniline is in low concentrations1.
Aniline can enter the body either by inhalation of air containing aniline, ingestion of food or water containing aniline, or by dermal contact with aniline. Inhalation of air containing aniline can cause respiratory tract irritation. Exposure to high levels can cause a number of adverse health effects including breathing difficulties, dizziness, headache, irregular heart-beat, methaemoglobinaemia (blood disorder), blue colour to the skin, convulsions and in extreme cases coma and death. Ingestion of aniline can lead to gastrointestinal irritation, nausea, vomiting and diarrhea. Exposure to high levels may lead to effects similar to those for inhalation. Dermal contact with aniline can cause skin irritation and sensitization. Absorption of large quantities of aniline through the skin may cause effects similar to those for inhalation. There is an increased risk in young infants of developing methaemoglobinaemia due to aniline exposure2.
Oxidation is an essential reaction for different organic synthesis. Chromium compounds have been used in aqueous and non-aqueous medium for the oxidation of a variety of organic compounds. Chromium compounds especially Cr(VI) reagents have been versatile reagents and capable of oxidizing almost all the oxidisible organic functional groups. The development of newer chromium (VI) reagents for the oxidation of organic substrates continues to be of interest3.In recent years, many such reagents have been developed with some success. Some new Cr(VI) based reagents like tetraheptylammonium bromochromate4, tripropyl ammonium fluorochromate5, isoquinoliniumbromochromate6, tetrahexylammonium bromochromate7, benzimidazolium fluorochromate8and tetrabutylammonium bromochromate9have been usedto study the oxidation of various organic compounds.
The oxidationkinetics of anilines by various oxidizing agents have been extensively studied10-14. Since anilines are very harmful to human health and the environment, removal of aniline from the environment is the ultimate goal of research today. For this a deep understanding of the mechanism of the oxidation process of aniline is needed. Furthermore, one of the important tools in deciding the mechanism of reactions is the study of substituent effects and thermodynamic parameters. The Hammett equation, also known as Linear Free Energy Relationships (LFER) have beenfound useful for correlating reaction rates and equilibrium constants for meta-and para-substituted derivatives of benzene. The isokinetic relationship is also an important tool for deciding the nature of a mechanism14. Keeping this in view, we report thekinetic features of the oxidation of a series of meta-substituted anilines by TBABC in anaqueous acetic acid medium to propose a suitable mechanism.
Experimental
Materials and Reagents
All the employed chemicals and solvents were of analytical grade. Anilines were used with substituents H, m-OCH3,m-OC2H5,m-CH3,m-F, m-Cl and m-NO2. Tetrabutylammoniumbromochromate (TBABC) was prepared by a reportedmethod9 and its purity was checked by the iodometric method. Doubly distilled water wasused for all purposes.The solid anilines were used as such and the liquid anilines were used after vacuum distillation. Acetic acid was purified by standard method and the fraction distilling at 118oC was collected.
Kinetic Measurements
The pseudo – first-order conditions were attained by maintaining a large excess ( x 15 or more) of aniline over TBABC. The solvent was 50% acetic acid – 50% water (v/v), unless specified otherwise. The reactions were followed, at constant temperatures (± 0.01 K), by monitoring the decrease in [TBABC] spectrophotometrically at 362 nm using UV–Vis spectrophotometer, Shimadzu UV-1800 model.
Data Analysis
Correlation analysis were carried out using Microcal Origin (Version 6.1) computer software. The goodness of the fit is discussed using the correlation coefficients and standard deviations.
Stoichiometry and Product Analysis
The stoichiomety of the reaction was determined by carrying out several sets of experiments with varying amounts of TBABC largely in excess over aniline. The estimation of unreacted TBABC showed that 1 mol of TBABC reacts with 1 mol of aniline. The oxidative products were analysed using preparative TLC on silica gel, which yields azobenzenem.p66oC (Lit 68oC) and UV Abs.(EtOH) at λmax320nm.
Results and Discussion
The oxidation of anilinesby TBABC has been conducted in 50% acetic acid and 50% water medium at 303K, under pseudo first order conditions and the result obtained were discussed in the following paragraphs.
The valuesof k1were independent of the initial concentration of TBABC (Table 1) suggesting the reactions were of first order with respect to TBABC. Thereaction was catalysed by hydrogen ions and the order with respect to [H+] wasone. Linear plots of log k1 versus log [Substrate] with unit slope demonstrate the first-order dependence of the rate on [Aniline].
The oxidation of aniline in a nitrogen atmosphere failed to induce the polymerizationof acrylonitrile. Furthermore, the rate of oxidation decreased with the additionof Mn(II), indicating the involvement of a two-electron reduction of Cr(VI)to Cr(IV)(Table 1). Therefore, a one-electron oxidation, giving rise to free radicals, is unlikely.
The oxidation of aniline has been studied in the binary mixture of acetic acid and water as the solvent medium. The concentration of acetic acid was varied from 30% to 70% and the rate were measured. The reaction rate increased remarkably with the increase in the proportion of acetic acid in the solvent medium(Table – 2). Positive slope of log k1 versus 1/D plot indicates that the reaction involves a cation-dipole type of interaction in the rate determining step15 (Fig. 1).
Solvent variations may affect the kinetics and the energy of the electrontransfer processes in a complex manner, particularly in mixed solvent media asthe physico-chemical properties of mixed solvent media are often quite differentfrom those of the pure solvents or of their ideal mixtures16. The dependence ofthe kinetic parameters for reactions on the composition of mixed aqueous solventsoften affords complicated patterns.
Rates of oxidation of anilines were determinedat different temperatures between 298 and 313 K in 50% – 50% (v/v) acetic acid – water medium in presence of perchloric acid and the pseudo-first order rate constants were determined (Table – 3). Various activation parameters were calculated and the values were presented in Table – 4. The entropy of activation is negative for anilines.
![Table 1: Effect of variation of [S], [TBABC] and [H+] on the rate of reaction at 303Ka](http://www.orientjchem.org/wp-content/uploads/2017/01/Vol33No1_Kine_SHAI_tab1-150x150.jpg) |
Table 1: Effect of variation of [S], [TBABC] and [H+] on the rate of reaction at 303Ka Click here to View table |
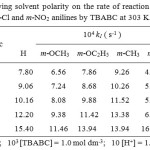 |
Table 2: Effect of varying solvent polarity on the rate of reaction of anilinem-OCH3, m-OC2H5, m-CH3, m-F, m-Cl and m-NO2 anilinesby TBABCat 303 K. Click here to View table |
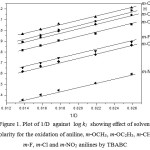 |
Figure 1: Showing plot of 1/D against log k1 showing effect of solvent polarity at various temperatures in the presence of oxalic acid Click here to View figure |
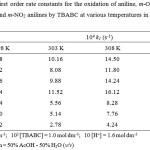 |
Table 3: Pseudo-first order rate constants for the oxidation of aniline, m-OCH3, m-OC2H5, m-CH3, m-F, m-Cl and m-NO2 anilinesby TBABC at various temperatures in aqueous acetic acid medium Click here to View table |
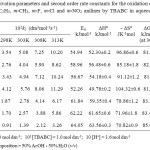 |
Table 4: Activation parameters and second order rate constants for the oxidation of aniline, m-OCH3, m-OC2H5, m-CH3, m-F, m-Cl and m-NO2 anilinesby TBABC in aqueous acetic acid medium Click here to View table |
The effect of structure on reactivity of meta-substituted anilines were studied. It is interesting to note that the reactivity decreases in the order m-CH3> H >m-OC2H5>m-OCH3>m-F >m-Cl>m-NO2for the substituents.
A linear Hammett’s plot is obtained when substituent constant (σ) for different substituents were plotted against log k2 for the oxidation of anilines by TEABC at various temperatures. The value of slope of Hammett plot is known as reaction constant (ρ). A linear Hammett’s plot is given in Figure 2. Reaction constant values at different temperatures are given in Table 5. According to Hammett, the reaction with positive ρ values are accelerated by electron withdrawal from benzene ring, whereas those with negative ρ values are retarded by electron withdrawal from benzene ring17. In this oxidation reactions, the electron withdrawing groups decreases the rate and the electron donating groups increases the rate. These observations supporting the negative ρ values obtained from the Hammett Plot. The negative ρ values further confirms the formation of a positively chargedtransition state.
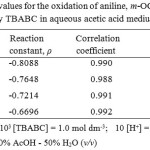 |
Table 5: Reaction constant values for the oxidation of aniline, m-OCH3, m-OC2H5, m-CH3, m-F, m-Cl and m-NO2 anilinesby TBABC in aqueous acetic acid medium at different temperatures |
 |
Figure 2: Showing Hammett plot of log k2 versus substituent constant σpfor the oxidation of aniline,m-OCH3, m-OC2H5, m-CH3, m-F, m-Cl and m-NO2 anilines by TBABC in aqueous acetic acid medium at different temperatures Click here to View figure |
Mechanism of oxidation
The sequence of reactions for the oxidation of anilines by TBABC in perchloric acid is shown in Scheme 1. The oxidation of anilines by TBABC in acetic acid water medium is remarkably slow, but is catalyzed in the presence of perchloric acid, and the reaction proceeds at a comfortable rate. Catalysis by perchloric acid suggests protonation of TBABC species rather than the aniline molecule, which would have resulted in retardation. The reaction did not promote polymerization of acrylonitrile indicating absence of free radicals. However, the addition of Mn (II), in the form of MnSO4, retards the rate of oxidation. This indicates the involvement of Cr(IV) intermediate in the oxidation of anilines by Cr(VI) reagent.
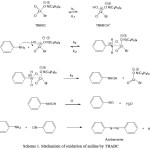 |
Scheme 1: Mechanism of oxidation of aniline by TBABC |
The reaction proceeds with the formation of a complex followed by the loss of hydride on. The complex is formed likely by concerted transfer of oxygen from the oxidant to N-atom and electron pair from N-atom to Cr (VI). The negative ρ value is indicative of the presence of a positive nitrogen center, which would mean depletion of lone-pair electron density, and this can be facilitated only when the oxidant forms a complex with the substrate in which the nitrogen lone-pair can be used up in coordinating with an electron-deficient center, preferably a metal ion. The above mechanism leads to the following rate law:
-d [TBABC] / dt = k1k2 k3[Aniline] [TBABC] [H+]
Conclusion
In this paper we have reported the detailed mechanism of oxidation of aniline and some para– substituted anilines by TBABC. The reaction is first order each in [Aniline], [TBABC] and [H+]. The oxidation of meta-substitutedanilines yield the corresponding azo benzenes. The negative ρ values obtained from the Hammett plot reveals that a positively charged reactive intermediate is formed during the oxidation process.Similarly the negative value of ∆S# provided support for the formation of activated complex in the slow step.
References
- Bhuvaneshwari, D.S.; Elango, K.P. Z. Naturforsch. 2006, 61b,1254 – 1260.
- Jin, Q.; Hu, Z.; Jin, Z.; Qiu, L.; Zhong, W.; Pan, Z. Bioresour. Technol. 2012, 117, 148-154.
CrossRef - Wiberg, W.B.Oxidation in Organic Chemisry, Academic Press, New York, 1995.
- Ghammamy, G.; Mehrani, K.; Afrand, H.; Javanshir, Z.; Rezaeibehbahani, G.R.; Moghimi, A.; Aghbolagh, Z. S.J. Chil. Chem. Soc.2009, 54, 491 – 494.
CrossRef - Yogananth, A.; Mansoor, S.S. Oriental J. Chem. 2015, 31, 17-23
- Vibhute, A. Y.; Patwari, S. B.; Khansole, S. V.; Vibhute, Y. B.Chin. Chem. Lett.2009, 20, 256 – 260
CrossRef - Mansoor, S.S.; Asghar, B.H. J. Indian. Chem. Soc. 2013, 90, 1395 – 1401.
- Malik, V. S.; Vannamuthu, I.; Shafi, S. S.; Mansoor, S. S. Oriental J. Chem.2015,31, 77-83
CrossRef - Ghammamy, G.; Mehrani,K.; Afrand, H.; Hajighahrammani, M. Afr. J. Pure Appl. Chem.2007, 1, 8 -10.
- Patwari, S.B.; Khansole, S.V.; Vibhute, Y.B. J. Iran. Chem. Soc. 2009, 6, 399-404.
CrossRef - Gupta,V.K. React. Catal. Lett. 1985, 27, 207- 211
CrossRef - Mansoor, S. S.; Shafi, S. S.Arab. J. Chem. 2014, 7, 171–176
CrossRef - Bhuvaneswari, D. S.; Elango, K. P. Int. J. Chem. Kinet. 2006, 38, 657–665
CrossRef - Bhuvaneshwari, D.S.; Elango, K.P. Z. Naturforsch. 2005, 60b,1105 – 1111
- Amis, E. S. Solvent Effects on Reaction Rates and Mechanisms. Academic Press, New York,1967, 42
- Bhuvaneshwari, D.S.; Elango, K.P. J. Serb. Chem. Soc.2008, 73 (7), 735–744
CrossRef - Hammett, L.P. Physical Organic Chemistry, First Ed. McGraw-Hill, New York, 1940

This work is licensed under a Creative Commons Attribution 4.0 International License.









