Amino Acid Derivative of Calix[4]Arene as Schiff Base for Sensing of Copper(II)
Mansoure Khanpour1, Saeed Taghvaei- Ganjali1 and Reza Zadmard2
1Department of Chemistry, Islamic Azad University, North Tehran Branch, P.O. Box: 15875-598, Tehran, Iran.
2Chemistry and Chemical Engineering Research Center of Iran, Tehran, Iran.
Corresponding Author E-mail: zadmard@ccerci.ac.ir
DOI : http://dx.doi.org/10.13005/ojc/330132
A new amino acid calix[4]arene derivative (5) has been designed, synthesized, characterized with different methods such as HRMS, NMR and FTIR. Our result shows that molecular receptor (5) is more proper for recognizing copper ion than other metal ions. In this paper we have developed a new sensor material for common ions such as copper at low concentration.
KEYWORDS:Calixarenes; Schiff bases; Cations
Download this article as:| Copy the following to cite this article: Khanpour M, Taghvaei-Ganjali S, Zadmard R. Amino Acid Derivative of Calix[4]Arene as Schiff Base for Sensing of Copper(II). Orient J Chem 2017;33(1). |
| Copy the following to cite this URL: Khanpour M, Taghvaei-Ganjali S, Zadmard R. Amino Acid Derivative of Calix[4]Arene as Schiff Base for Sensing of Copper(II). Orient J Chem 2017;33(1). Available from: http://www.orientjchem.org/?p=30243 |
Introduction
Calixarenes are cyclic oligomer which have been applied as ionic and molecular recognition in the past years [1].
The Calixarene chemists have highly noticed selective proteins, anionic or cationic sensing by changing in quenching effect on fluorescence as a result of its relative ease in addition to high sensitivity and selectivity [2,3].
The development of chemosensors for recognizing copper ions has attracted a great deal of interest thanks to its vital role in chemical and biological systems [4,5]. Disproportionate amounts of copper ions could possibly result in neurodegenerative diseases and amyotro- phiclateral sclerosis [6]. Different Methods such as UV, near infrared and emission spectroscopies were developed for sensing of copper ions using and sensing mechanisms engaging photoinduced electron transfer,
fluorescence resonance energy transfer, internal charge transfer, nanotechnology and other photophysical properties which are compatible with the analysis of Cu2+ in biological systems [7].
It is extremely popular that calixarens can convert to other conformations very fast [8]. If the desired function is protein or cationic and anionic recognition, the molecular receptor which has been synthesized, have a prudent balance between flexibility and rigidity. So that it can interact with the different guests. Due to the rigid Calix [4] arene platform and its small cavity, it is not able to accommodating different guest. Calixarenes macrocycle could be modified by functionalization at their upper or the lower rim [9].
Most reported chemosensors for copper ions contain the attachment of fluorophore units to the macrocyclic or chelating scaffolds. Among famous macrocyclic frameworks, calix [4] arenes are known to be highly useful building for achieving ionic and molecular recognition [10], possibly because of their preorganized cavity and facile modifiability.
Schiff bases (imines) are also known to serve as good chelating sites for metal ions [11]. Hence, a molecular probe that has a calixarene scaffold, fluorescence moiety and a Schiff base substructure seems to be worth examining for recognizing metal ions [12].
Here we explain the synthesis of new Schiff-base calix[4]arene receptor with various functional units for the identification of copper ion in acetonitrile solution.
Materials and Methods
We have used commercial quality solvents and reagents. All cation solutions were prepared from their perchlorate salts and solution of Cu+ was also provided from CuI salt.
1H, 13C NMR spectra were recorded on a Bruker Avance spectrometer at 500 and 125 MHz using CDCl3 with TMS reference. In order to record infrared spectra¸ a Galaxy series FT-IR 5000 spectrophotometer was applied. The melting points were measured by using of a Buchi B-545 apparatus (uncorrected). A Qstare ERSI-q- TOF was used to recording of High resolution mass spectra (HRMS). Florescence spectra were recorded on JASCO FP-6500.
Synthesis Compounds (1- 3) were prepared based on the literature methods [13, 14]. The synthesis of other compounds (4-5) was done by adjusting known synthetic procedures.
 |
Figure S1: 1H NMR (400 MHz, CDCl3) spectrum of 4 |
25,26,27-Tripropoxy-28-[(3-(4-formylphenoxy)propoxy)]-5,11,17,23tetra (tertbutyl)calix[4]arene (4, C63H84O6)
p-hydroxybenzaldehyde 0.23 g (1.92 mmol) and a mixture of 3, 0.9 g (1 mmol) and K2CO3, 0.43 g (3.11mmol) dissolved in acetonitrile (50 mL), and the solution was stirred for 60 hours were refluxed. After cooling, the solvent evaporated under vacuum. The residue solid was extracted with CH2Cl2 (100 mL) and rinsed off with water (50 mL) and 1 M HCl (2 × 50 mL). Then the organic layer was dried over MgSO4 and crystallized from CH2Cl2: methanol (1:1). Yield 0.84g (85%) 4. Tmp.: 211–213ºC;IR, ν, cm−1: 1667 (C=O) ; 1H NMR, δ, ppm: δ= 0.93t (6H, CH3), 1.02 s (18H, -C(CH3)3), 1.05 s (9H, -C(CH3)3), 1.2 s (9H, -C(CH3)3), 1.3 t(3H, CH3), 2.01-2.07m (6H, -OCH2-CH2-CH3-), 2.67 m(2H, -OCH2-CH2-CH2O-), 3.18 d (4H, J = 12.5 Hz, ArCH2Ar), 3.82 t (4H, -OCH2-CH2-CH2-),3.93 t(2H, -OCH2), 4.15 t (2H, -OCH2), 4.31t (2H, -OCH2), 4.35 d (4H, 2J = 12.5 Hz, ArCH2Ar), 6.74 d (4H, J = 2.0 Hz, ArH), 6.92 s (4H, ArH), 7.08 d (2H, J = 2.0 Hz, ArH), 7.89 d(2J = 11.9 Hz, 2H, ArH), 9.94 s (H, CHO); 13C NMR (125 MHz) δ= 10.71, 10.85, 23.69, 23.82, 30.37, 31.47, 31.50, 31.80, 31.95, 31.98, 34.17, 34.30, 66.61, 72.08, 72.21, 77.72, 115.14, 125.15, 125.32, 125.47, 125.59, 132.43, 133.55, 133.76, 134.71, 144.63, 144.75, 145.19, 153.70, 164.71, 191.21; HRMS m/z= (951.47) [M]+
 |
Figure S2: 13C NMR (100 MHz, CDCl3) spectrum of 4 |
25,26,27-Tripropoxy-28-[(3-[(methyl 2-(benzyliden amino) propanoate)]propoxy)]-5,11,17,23 tetra(tertbutyl)calix[4]arene (5, C67H91NO7)
A solution of L-Alanine methyl ester hydrochloride 0.04 g (0.37 mmol) and triethylamine (1 mL, excess) in MeOH (10 mL) were added to solution of compound 4 0.2 g (0.37 mmol) in CHCl3 (50 mL) and the reaction mixture was refluxed for 2 days. After cooling the crude material was crystallized from CHCl3: MeOH (1:3). Yield 0.7g ( 71%) 5. Tmp.:110–114oC IR, ν, cm−1: 1750 (C=O); 1H NMR, δ, ppm: δ= 1.02 s (9H, -C(CH3)3), δ= 1.04s (18H, -C(CH3)3), 1.05-1.12 m (18H, -C(CH3)3 and CH3) ), 1.22 d (3H, CH3), 1.55 m (6H, -OCH2-CH2-CH3-), 2.07 m(2H, -OCH2-CH2-CH2O-), 3.14 d (4H, J = 12.3 Hz, ArCH2Ar), 3.72-4.05 m (14H, -OCH2-CH2-CH2-, OCH3, CH-CH3), 4.40 d (4H, 2J = 12.3 Hz, ArCH2Ar), 6.68-6.94 m (13H, ArH and CH); HRMS m/z= (1055.6644) [M]+
 |
Figure S3: FT-IR spectrum of 4 |
Results and Discussion
In this work we synthesized new chiral Schiff base calix[4]arene and also investigated identification properties of it towards different metal ions. The synthesis of 5 is depicted in Figure 1.
To prepare the 4¸ p-hydroxybenzaldehyde was added to 3 in CH3CN in presence of K2CO3 as a base. Compound 4 was characterized by a combination of FT-IR, 1H NMR and HRMS. The spectral data of 5 could be found in the experimental section. Compound 5 is asymmetric because of the connection of amino acid as a chiral subunits in the calix[4]arene.
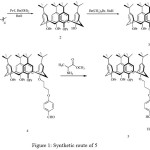 |
Figure 1: Synthetic route of 5 |
 |
Figure S4: High-resolution mass spectrum (HRMS) of 4 Click here to View figure |
Selectivity and sensitivity of the synthesized receptor 5 were studied by using fluorescence titration experiments in CH3CN solution. The Data analysis and Ka values were determined by Sigmaplot-software. Our results show that receptor 5can be served as a selective metal sensors. Figure 3 illustrates how adding a number of metal ions can change the fluorescence intensity of molecular receptor 5 relatively. From this data, it can be observed that whereas no remarkable change occurs by adding number of metal ions, Cu2+ generates a significant quenching on its emission band. This could be applied as a fluorescence sensor which needed a complete investigation of 5 through fluorescence spectroscopy when different ions are present. fluorescence emission spectrum of 5 was recorded from 300 to 550 nm by exciting it at 290 nm when the emission maximum was recorded at 330 nm.
Binding constants (Ka) were calculated by equation based on literature [15].
The fluorescence intensity of 5 in the presence increasing concentration of Cu2+ is shown in Figure2. According to our fluorescence spectral studies the association constant between 5 and Cu2+ was 1.2 × 105 M−1.
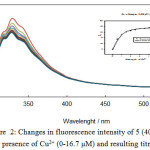 |
Figure 2: Changes in fluorescence intensity of 5 (40 µM) in ACN in presence of Cu2+ (0-16.7 µM) and resulting titration curve |
 |
Figure S5: 1H NMR (400 MHz, CDCl3) spectrum of 5 |
More over¸ In order to understand the selectivity Cu2+ by the receptor¸ comparative cations fluorescence measurements were done with different cations. It has been found that Cu2+ does confer significant change in the fluorescence measurements. (Figure 3)
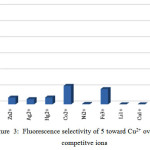 |
Figure 3: Fluorescence selectivity of 5 toward Cu2+ over various competitve ions |
The Job’s plot studies show a 1:1 stoichiometry between (5) and Cu2+ion.
In order to examine the interaction between Schiff base moiety 6 (Figure 4) and different ions metal, this compound was prepared based on the literature methods [16]. Our results show that only the Schiff base moiety has no interaction with the metal ions.
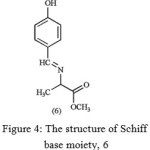 |
Figure 4: The structure of Schiff base moiety, 6 |
 |
Figure S6: High-resolution mass spectrum (HRMS) of 5 |
Acknowledgements
Partial financial support by Chemistry and Chemical Engineering Research Center of Iran is greatly appreciated.
References
- Chawla, H.M.; Pant, N.; Kumar, S.; Kumar, N.; Black david, St.C. Calixarene-based materials for chemical sensors. In: Korotcenkov, G., (Eds.), Chemical Sensors: Fundamentals of Sensing Materials. Polymers and other materials. Momentum Press, 2010, 3, pp. 300, New York.
- Kumar, A.; Kumar, V.; Upadhyay, K.K.; Roychowdhury, P.K.J. J. Mol. Struct. 2013, 1035, 174-182.
CrossRef - Kim, H.J.; Kim, S.H.; Anh, L.N.; Lee, J.H.; Lee, C.; Kim, J.S. Tetrahedron Lett. 2009, 50, 2782-2786.
CrossRef - de Silva, A.P.; Fox, D.B.; Huxley, A.J.M.; Moody, T.S. Coord. Chem. Rev. 2000, 205, 41-57.
CrossRef - Que, E.L.; Domaille, D.W.; Chang, C.J. Chem. Rev. 2008, 108, 1517-1549.
CrossRef - Gaggelli, E.; Kozlowski, H.; Valensin, D.; Valensin, G. Chem. Rev. 2006, 106, 1995-2044.
CrossRef - Schaferling, M. Angew. Chem. Int. Ed. 2012, 51, 3532-3554.
CrossRef - Chawla, H.M.; Hundal, G.; Singh, S.P.; Upreti, S. CrystEngComm. 2007, 9, 119-122.
CrossRef - Gutsche, C.D. Calixarenes: Monographs in Supramolecular Chemistry, in: Stoddart, J.F. (Eds), Edu. Div. RSC. 1989, London.
- Chawla, H.M.; Shukla, R.; Pandey, S. Tetrahedron Lett. 2012, 53, 2996-2999.
CrossRef - Xu, V.; meng, J.; Meng, L.; Dang, Y.; Cheng, Y.; Zhu, C. Chem. Eur. J. 2010, 16, 12898-12903.
CrossRef - Hsieh, W.H.; Wan, C.F.; Liao, D.J.; Wu, A.T. Tetrahedron Lett. 2012, 53, 5848-5851.
CrossRef - Gutsche, C.D.; Iqbal, M.; Stewart, D. J. Org. Chem. 1986, 51, 742-745.
CrossRef - Iwamoto, K.; Araki, K.; Shikai, S. J. Org. Chem. 1991, 56, 4955-4962.
CrossRef - Inoue, Y.; Yamamoto, K.; Wada, T.; Everitt, S.; Gao, X.M.; Hou, Z.J.; Tong, L.H.; Jiang, S.K.; Wu, H.M. J. Chem. Soc. Perkin Trans. 2. 1998, 1807-1816.
CrossRef - Dios, A.; Mitchell, R.A.; Aljabari, B.; Jodi, L.; O‘Connor, K.A.; Liao, H.; Senter, P.D.; Manogue, K.R.; Lolis, E.; Metz, C.; Bucala, R.; Callaway, D.J.E.; Al-Abed, Y. J. Med. Chem. 2002, 45, 2410-2416.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









