The Solvent Extraction of Cadmium From Phosphoric Acid Solution By3-Methyl-Quinoxaline-2-Thione in Toluene Diluent
Sanae Baki Senhaji1*, Ahmed Elyahyaoui1, Saidati Boulassa1 and El Mokhtar Essassi2
1Laboratory of Radiochemistry, Department of Chemistry, Faculty of Sciences-Rabat, Morocco.
2Laboratory of heterocyclic organic chemistry, Faculty of Sciences, Rabat, Morocco.
Corresponding Author E-mail: b.senhaji.sanae@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320624
The solvent extraction of cadmium (II) from phosphoric acid 2.5M by 3-methyl-2 (1H)-quinoxaline-thione
![]() is investigated in various experimental conditions. Obtained results show a variation of extraction of Cd with pH, and
is investigated in various experimental conditions. Obtained results show a variation of extraction of Cd with pH, and
![]() concentration.
The conventional methods of slope analysis can’t be performed successfully for understanding interaction mechanisms. A technique combining slope analysis with the variation of overall extraction constant with pH is performed in this case. There is no predominant removal mechanism, cadmium is extracted as complexes in
concentration.
The conventional methods of slope analysis can’t be performed successfully for understanding interaction mechanisms. A technique combining slope analysis with the variation of overall extraction constant with pH is performed in this case. There is no predominant removal mechanism, cadmium is extracted as complexes in
![]() stoichiometry varying between 1:1:0 and 1:3:3.
The extraction reaction is a complex process including ion exchange-ion pair formation,organic and inorganic complexation, H3PO4deprotonation and solvatation phenomena. A best recovery of 43 to 60% is achieved in acidic conditions with CLH = 0.26M
stoichiometry varying between 1:1:0 and 1:3:3.
The extraction reaction is a complex process including ion exchange-ion pair formation,organic and inorganic complexation, H3PO4deprotonation and solvatation phenomena. A best recovery of 43 to 60% is achieved in acidic conditions with CLH = 0.26M
Cadmium; extraction; phosphoric medium; pH; 3-methyl-2 (1H)-quinoxaline-thione
Download this article as:| Copy the following to cite this article: Senhaji S. B, Elyahyaoui A, Boulassa S, Essassi E. M. The Solvent Extraction of Cadmium From Phosphoric Acid Solution By3-Methyl-Quinoxaline-2-Thione in Toluene Diluent. Orient J Chem 2016;32(6). |
| Copy the following to cite this URL: Senhaji S. B, Elyahyaoui A, Boulassa S, Essassi E. M. The Solvent Extraction of Cadmium From Phosphoric Acid Solution By3-Methyl-Quinoxaline-2-Thione in Toluene Diluent. Orient J Chem 2016;32(6). Available from: http://www.orientjchem.org/?p=25516 |
Introduction
Due to their harmful effects, heavy metals are a major threat to ecological environment, food safety and agriculture sustainable development. As a result, these elements are not suitable in nature that various measures are undertaken to control, and prevent their presence in environment. Among these heavy metals, cadmium is listed as the seventh most toxic substances [1-2].
Enrichment of Cd in soils is reporte , through the land application of bio solids, industrial effluents, and Cd-rich phosphate fertilizers [3]. In general, phosphate fertilizers are produced by the acidulation of crushed and powdered phosphate.
Thus the removal of Cd(II) from phosphoric acid which is the intermediary product for the production of all high grade phosphate fertilizers, is of ever-increasing interest. Several methods including precipitation, solvent extraction and ion exchange are proposed in this case.
Amine compounds are successfully used for cadmium separation from complex aqueous media. However, in this extraction system, metal extraction is carried out from acidic media, so that the amine in the organic phase is first converted to its salt. To avoid this limitation on the aqueous condition, quaternary ammonium salts, is employed in neutral, and slightly alkaline. As known the sulfhydryl-containing compounds have the ability to chelate heavy metals such cadmium that Chelating exchangers with thiol functional groups are used for Cd(II) removal from aqueous solutions [4-5].
Recently the use of extractants with a bifunctional groups such asquinoxaline-thione for zinc and cadmium removal are highlighted as potential extractant for metal removal from complex aqueous media [6-7].
The advantage of this process is that can combine organic and inorganic complexing phenomena, ion-pair formation and ion exchange reactions. Thus, cadmium removal from H3PO4 3.22 M is successfully achieved over wide range of pH, in this case. Understanding interaction mechanisms involved in this case, is essential and allows efficient use of solvent extraction procedure[8].
This interaction is also an actual problem of modern analytical chemistry, which is primordial for environmental sciences, as well as geology, clinical and medicinal chemistry, as discussed previously [9].
Unfortunately, it is reported that Cd(II) extraction by amines is a variable phenomenon when inorganic complexation is involved in aqueous phase that slope analysis cannot be used to carry out extraction stoechiometry [10] . Similar results are obtained for this metal in phosphoric medium [7]. To determine extracted species, a technique combining slope analysis with the variation of apparent extraction constant with pH is performed, in this case. As shown, cadmium removal is essentially achieved via anion-exchange. Obviously to control extraction process in question, more informations are required for the removal of Cd with analytical concentration variation of H3PO4.
For these reasons the present study aims to carry out cadmium extraction reaction predominating in H3PO4 2.5M.
Material and Methods
Reagents
All reagents used: Cd(NO3)2 , HCl, NaOH, and H3PO4 are of analytical grade and used without further purification. Stock solutions (10-3mol/l) of Cd(II) are prepared by dissolving known amount of cadmium nitrate in adequate volume of 0.1M laboratory phosphoric acid. Initial pH of the aqueous phases, is adjusted using 0.1M KOH or 0.1M HNO3, and measured before and after extraction test.
Procedure
The organic and aqueous solutions (H3PO4 2.5M) were equilibrated in 20 ml separator funnel with an organic/aqueous ratio of 1:1 (5 ml organic solution and 5 ml aqueous solution) which is shaken vigorously for 15min. Preliminary tests have shown that equilibrium of extraction is reached in less than 5 min. After equilibration, the separator funnel is left standing for at least 30 min for completing phase separation. After equilibration, the separator funnel is left standing for at least 30 min for completing phase separation. The synthesis of 3-methyl-2 (1H)-quinoxaline-thione (C9H8N2S) designated thereafter as
![]()
is performed as described previously [6-7]. A solution of synthesized extractant is prepared with dissolution of 0.26 mol/l of
![]()
in toluene (C7H8).
The extraction of cadmium is studied from aqueous phosphoric acid solutions (2.5M) containing 10-4M Cd(II). All the extraction experiments were carried out at room temperature.
The Cd(II) concentrations in the aqueous phase
![]()
were determined using Benchtop XRF Spectrometer with X-LabPro Version 4.0 software. While the concentration of cadmium in the organic phase
![]()
was calculated from material balance.
The distribution ratio, D, is calculated as follows:
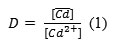
Results and Discussion
Effect of Equilibrium pH
Fig. 1 reproduces the logarithmic variation of D with pH for H3PO4 2.5M, in the pH range of about 1.7 to 7.0, and analytical ligand concentration
![]()
and 0.62M. As can be seen, extraction of cadmium increases with increase in pH to reach a maximum value at pH = 2.1, and after a steady stage observed in the pH range 2.1 – 3.4, logD increases as pH continue to rise. Maxima are found at pH 3.7 and 4.5, in this case. Extraction yield higher than 66% is achieved at
![]()
for pH ranging from 2.0 to 5.0. As discussed previously, distinct pH segments with continuous varying slopes suggest that cadmium extraction rises from different reactions [7].
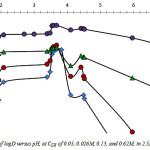 |
Figure 1: Variation of logD versus pH, at of 0.05, 0.026M, 0.13, and 0.62M, in 2.5M H3PO4. |
Effect of Extractant Concentration
Fig.2 reproduces the logarithmic variation of D with
![]()
at different pH values, for H3PO4 2.5M
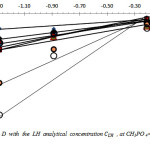 |
Figure 2: Variation of log D with the LH analytical concentration CLH , at CH3PO 4= 2.5M, for pH value ranging from 1.5 to 6.7. Click here to View figure |
As found, these variations are linear with slopes varying in the range of about 1 to 6. The overall stoechiometry extraction reaction is not an integer as could be expected from theoretical single reaction. This indicates a variation of extraction of Cd with pH, and LH concentration. Similar results are observed previously for the extraction of cadmium, zinc and some rare earths by various chelatants [6-7; 10-14]. This shows that at least, two predominant extracted complexes are involved in the removal of Cd (II), in this case.
Cadmium Extraction from Phosphoric Acid Solutions
From results obtained previously for Cd(II) removal by 3-methyl-quinoxaline-2-thione [6-7], the extraction equilibrium from phosphoric media may be written in the general forms:
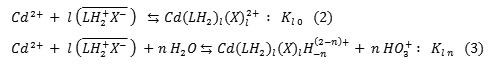
Where X– is aqueous anionic ligand, the onlined entities refer to organic phase, and n = 0;1 ;2, etc….The symbol H-n stands both for hydrogen (n<0) and for OH group (n>0). Kl n denotes the constant of equilibrium reaction. The extracted species are given by:
![]()
Kl n is given by :
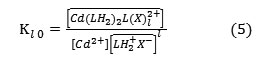
and
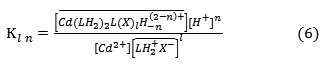
Assuming in first approximation that
![]()
which results in
![]()
we obtain:
![]()
One can note that n=0 for ion-pair formation.
The nature of C1n extracted species noted thereafter (l, n) can be obtained from logD=f
![]()
variations according to modified slops method:
![]()
and
![]()
The parameters n and l are varying with experimental conditions. As indicated above there is no predominant extraction reaction, that Cd extracted specie often results in combining two entities [6-7]. Conventional methods of slope analysis can’t be performed successfully in this condition [10; 13].In this case numerical modeling procedure is performed by fitting experiment data to a polynomial equation [7].
Fig. 3 shows plots of and
![]()
and
![]()
as a function of pH. As shown, the molar ratio metal: LH: H3O+are varying continuously in extracted entities with aqueous solution acidity. Overall equilibrium constant are obtained from the origin ordinates of log
![]()
given by A = logK1n + n pH.
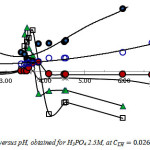 |
Figure 3: Variations of l, n, and A versus pH, obtained for H3PO4 2.5M, at CLH = 0.026, 0.052, 0.13 and 0.62M. Click here to View figure |
From these results the extraction reaction is found to be influenced by both extractant concentration and aqueous media acidity. The mechanism ofthis reaction involves deprotonation in more acidic condition (pH < 2) and for pH ranging from 3.4 to 4.1. Under mild acidic medium (pH 2.2 – 3.3) recovery proceeds entirely by no ion exchange, in all the cases, and also for
![]()
M, at pH > 4.1. While in soft acidic conditions (4.1 < pH < 7), OH– exchange is prevalent, for
![]()
and 0.13M.
Evaluation of Kl, n
Table I show, as example, some overall and predominant extraction constants.
Table I: Equilibrium logarithmic constants (logKln) of cadmium extraction by 3-Methyl Quinoxaline-2-thione from H3PO4 2.5M. . (* Calculated constant)
|
(l ;n) |
logKln |
|
(l ; n) |
logKln |
|
(2.71; 1.01) |
-1.06 |
(1 ; 1) |
2.71* |
|
|
(2.68; 1) |
-0.89 |
(1 ; 2) |
-6.73* |
|
|
(2.68; 1.83) |
-2.58 |
(1 ; 3) |
-8.79* |
|
|
(2.68; 2.88) |
-4.60 |
(2.74;-0.57) |
1.50 |
|
|
(2; 0.04) |
0.66 |
(2.74;-1.71 |
8.92 |
|
|
(1.6; 0.04) |
0.54 |
(2.74;-2.86 |
14.08 |
|
|
(1,6; 0.56) |
-1,27 |
(3 ;-0.57) |
3.82 |
|
|
(1.6; 2.36) |
-7,54 |
(3 ;-1.7 |
8.93 |
|
|
(2 ; 1) |
0.76* |
(3;-2.8) |
13.90 |
|
|
(2 ; 2) |
-5.63* |
(3, 0) |
1.23* |
|
|
(2 ; 3) |
-13.66* |
(3, 1) |
-1.72* |
|
|
(2, -1) |
4.53 * |
(3, 2) |
-0.73* |
|
|
(2, -2) |
8.12* |
(3, 3) |
-1.67* |
|
|
(2, -3) |
10.53* |
(3,-1) |
5.8* |
|
|
(1.0; 0.0) |
0.77 |
(3,-2) |
10.3* |
|
|
(1.0; 2.2) |
-7.12 |
(3,-3) |
14.8* |
|
|
(1; 0.75) |
-2 |
(1 ;2) |
-6.73* |
|
|
(1; 1.5) |
-4.76 |
(1 ;3) |
-8.79* |
It is interesting to indicate that l and │n│ represent successively the ligand number involved in extracted specie, and the predominant H+or OH– exchange reactions, contributing to overall equilibrium. Thus the overall constant of extracted complex (l, n), combining for successive (l’, i) , (l’, j), and (l’’, i) (l’’, j) species is given by:
Kl ;n = ( Kl’; n)(1 – x)(Kl”; n)x (10)
With l’< l <l”, l”= l’ +1, and x = l –l’ ,
and Kl ;n = ( Kl; i)(1 – y)(Kl; j)y (11)
with | i | < | n | < | j | and |j |= | i | +1, and y =|n | – |i |
and Kl; i = ( Kl’ ; i )(1 – x)(Kl’’; j )x (12), Kl; j = ( Kl’ ; i )(1 – x)(Kl’’; j )x (13)
Kl ;n = ( Kl’; i)(1-x)(1 – y)(Kl’; j)y(1 – x)° ( Kl’’; i) x(1 – y) ( Kl’’; j) x y . (14)
This allows to obtain :
logKl ;n ==(1-x) ( 1 –y) logKl’ ;i + y(1-x) logKl’ ;j+ x( 1 –y) logKl’’;i+ x( y) logKl ‘’ ;j (15)
As example, the overall partition equilibrium (2.68; 1.83) in the pH range 1.9 to 2, involves successive extracted species(2; 1), (2; 2), (3; 1), and (3; 2). Taking into account the protonation of amino group at pH <3 [6-7], and percentage distribution of phosphoric complexes of cadmium versus pH[15], predominant reactions combing, in this case, in overall extraction are as follows:

The equilibrium constants for the individual reactions could be obtained from the overall equilibrium constant Klnaccording to:
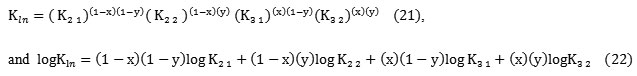
As example, in the case of (2.68; 1.83) complex we have:
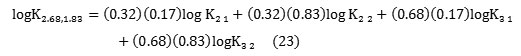
Conclusion
3-methyl-2 (1H)- Methyl Quinoxaline-2-thione
![]()
is used for the extraction for cadmium from phosphoric acid (2.5 M). This extraction is varying with initial organic ligand concentration and pH. The stoichiometry of the extracted Cd(II) complex isvarying between 1:1:0 and 1:3:3:
![]()
This stoichiometry was determinedby a modified slope analysis. The extraction reaction is a complex process including ion exchange-ion pair formation,organic and inorganic complexation, H3PO4deprotonation and solvatation phenomena. A best recovery of 43 to 60% is achieved in acidic conditions with CLH=0.26M
References
- JaishankarM., TsetenT., AnbalaganN., MathewB. B.: Toxicol. 2014, 7(2), 60–72.
- ZhitongY., Jinhui L., HenghuaX. ,Conghai Y.: Procedia Environmental Sciences, 2012,16, 722 – 729.
CrossRef - CiadamidaroL., SchulinR., MadejonP. , ́ Reiser R., Clucas L., Weber P., Robinson B. : Environ. Sci. Technol,2013, 47, 4497−4504.
- HynekD., KrejcovaL., SochorJ., CerneiN., KynickyJ., AdamV., TrnkovaL. , HubalekJ. , VrbaR. ,KizekR.:Int. J. Electrochem. Sci., 2012, 7, 1802 – 1819.
- HubickiZ., KołodyńskaD. : Ion Exchange Technologies. Maria Curie-SkłodowskaUniversityPoland.2012.
- SenhajiS. B., ElyahyaouiA. : Orient. J. Chem, 2015, 31 (3), 1601-1609.
CrossRef - SenhajiS. B., ElyahyaouiA., SaidatiB., EssassiE. : IJSR, 2016, 5 (9) , 6-15.
- Lijuan W.: B.Sc.Victoria, University of Technology Victoria, Australia, 1998.
- RomanovaT.E. , ShuvaevaO.V.: Chemical Speciation & Bioavailability, 2015,27(3), 139-145.
CrossRef - RiceN.M., SmithM. R.: J. appl. Chern. Biotechnol, 1975, 25, 379-402.
- StewartJ.A. : Rare Earth Production and Characterization Studies , University of Tennessee, Knoxville , 2015.
- NKPOZI, solvent extraction studies,University of Nigeria Nsukka, 2015.
- Onghena, BinnemansK., pure and industrial chemistry studies,University of Nigeria Nsukka, 2015.
- Onghena and KoenBinnemans, Ind. Eng. Chem. Res, 2015, 54, 1887−1898.
CrossRef - ElyayaouiA., BoulhassaS., GuillaumontR.: Journal of Radioanalytical and Nuclear Chemistry, 1990, 142(2), 403-415.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









