The Effect of Calcination Temperatures on the Activity of CaO and CaO/SiO2 Heterogeneous Catalyst for Transesterification of Rubber Seed Oil in the Presence of Coconut Oil as A Co-Reactant
Kamisah D. Pandiangan1,2*, Novesar Jamarun2*, Syukri Arief2, Wasinton Simanjuntak1 and Mita Rilyanti1
1Department of Chemistry, Andalas University, Padang, Indonesia.
2Department of Chemistry, Lampung University, Bandar Lampung, Indonesia.
Corresponding Author E-mail: kamisahdelilawati@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320622
This study was carried out to examine the potential of conversion of rubber seed oil into biodiesel. A series experiments was conducted using CaO and CaO/SiO2 as catalyst and coconut oil as co-reactant, with the main purpose to assess the effect of calcination temperatures on the performance of the catalysts. The results obtained demonstrate that the use of coconut oil as co-reactant significantly promoted conversion of fatty acids in rubber seed oil into their corresponding methylesters. It was also found that the catalytic activity of both CaO and CaO/SiO2 was significantly influenced by calcination temperatures and at the same temperature, CaO/SiO2 exhibited higher activity than unsupported CaO. The highest yield was achieved using CaO/SiO2catalyst subjected to calcination at 800 oC
KEYWORDS:rubber seed oil; transesterification; heterogenous catalyst; co-reactant
Download this article as:| Copy the following to cite this article: Pandiangan K. D, Jamarun N, Arief S, Simanjuntak W, Rilyanti M. The Effect of Calcination Temperatures on the Activity of CaO and CaO/SiO2 Heterogeneous Catalyst for Transesterification of Rubber Seed Oil in the Presence of Coconut Oil as A Co-Reactant. Orient J Chem 2016;32(6). |
| Copy the following to cite this URL: Pandiangan K. D, Jamarun N, Arief S, Simanjuntak W, Rilyanti M. The Effect of Calcination Temperatures on the Activity of CaO and CaO/SiO2 Heterogeneous Catalyst for Transesterification of Rubber Seed Oil in the Presence of Coconut Oil as A Co-Reactant. Orient J Chem 2016;32(6). Available from: http://www.orientjchem.org/?p=26263 |
Introduction
In response to increased need for renewable energysource, liquid fuel in particular, biodiesel continues to gain progressive share in the current global energy market. However, it should also be acknowledged that relatively higher price than that of petrol-diesel and competition between food and energy as a result of biodiesel industry reliance on edible vegetable oils have not been completely resolved up to present. From the raw material point of view, the use of non- edible vegetable oils is an ideal solution since many non-edible vegetable oil producing plants are known and can be found in different parts of the world.
One of the vegetable oil producing plants cultivated in many tropical countries, including Indonesia, is rubber (Hevea brasiliensis). The seed of this plant contains vegetable oil in substantial amount, ranging from 40 to 59%1-2, which makes this non-edible vegetable oil an interesting alternative raw material for biodiesel production. Despite this potential, unlike those derived from other raw materials, biodiesel derived from rubber seed oil has not reached the commercial stage. The main reason is probably due to the facts that rubber seed oil is more difficult to convert into biodiesel as a result of high free fatty acid (FFA) content in the oil which may reaches 18%3. In previous study4 it was reported that the large quantity of FFA in vegetable oil led to simultaneous saponification and transesterification reaction.
In an attempt to circumvent this technical difficulty arises from the FFA present, separation of the FFA using steam distillation and extraction by alcohol have been proposed5-6. However, steam distillation requires high temperature and has low efficiency, while extraction needs a large amount of alcohol because of the limited solubility of FFAs in alcohol. In this current study, utilization of coconut oil as a co-reactant was proposed as another approach, since coconut oil is known as an easily converted vegetable oil into biodiesel. In our previous study7, this approach was found to significantly promoted transesterification of castor oil, and therefore it is presumed that this approach will similarly work for rubber seed oil.
In this study, a series of transesterification experiments was carried out using heterogeneous catalyst, CaO/SiO2 (1:3, mass ratio), prepared from rice husk silica and CaO using sol-gel technique. The CaO was chosen since many studies have revealed that this oxide exhibited high catalytic activity for transesterification reaction8-9. In addition CaO is very cheap, non-toxic, has low solubility in methanol10, and can be obtained from various sources with simple method11. Before use, the catalyst was subjected to calcination treatment at different temperatures of 500, 600, 700, 800, and 900 oC for six hours.
Materials and Method
Reagent grade sodium hydroxide, nitric acid,methanol, and n-hexane, were purchased from Aldrich. Reagent grade methanol was purchased from Merck. Calcium oxidewas obtained from a local company in the City of Padang, West Sumatera. Rice husk was obtained from local rice milling industry in the City of Bandar Lampung. Before use, the husk was soaked in distilled water overnight, and sinking husk presumed to contain silica in high quantity, was collected, while floating husk was discharged. The main equipments used in this study are XRF PANalytical Epsilon 3 for elemental composition of the catalyst, and GC-MSQP2010 SE SHIMADZU for identification of transesterification product.
Extraction of Rubber Seed Oil
Rubber seed oil was mechanically extracted using the DL-ZYJ02 oil press machine. Rubber seed kernel was removed from the hull and cut into four pieces. The kernel was oven dried at 115 oC for 10 hours to expel the water, and dried kernel was subjected to extraction.
Extraction of Rice Husk Silica
Rice husk silica was obtained using an alkali extraction method, as previously applied7,12. Typically, 50 g dried husk sample wasplaced in a beaker glass, and an aliquot of 500 mL of 5% NaOH solution was added into the beaker. To commence the extraction, the mixture was heated to boil and left for 30 min. The mixture was left overnight at room temperature, and then filtered. The filtrate which contains silica (silica sol) was collected and then neutralized by dropwise addition of 10% HNO3 solution until the sol was converted into gel. The gel was aged for three days, and then rinsed repeatedly with de-ionized water to remove the excess of acid. The gel was oven dried at 110 oC for eight hours and ground into powder.
Preparation of CaO/SiO2
Preparation of CaO/SiO2 catalyst was carried out using sol- gel technique13-15.A sample of 21.0 g of silica powder was re-dissolved in 600 mL of 1.5% NaOH solution and 7.0 gof CaO was dissolved in 10 mL concentrated HNO3. The two solutions were mixed by slow addition of CaO solution into silica sol under stirring and then left until the mixture transformed into gel. The gel was then oven dried at 110 oC and ground into powder, and divided into five samples for calcination treatment at different temperatures of 500, 600, 700, 800 and 900 oC. The sample was calcined using temperature program with the heating rate of 5 oC/min, and holding time of 6 hours at peak temperature.
Transesterification Experiment
Each of the catalysts was tested in transesterification experiment with the purpose to investigate the effect calcination temperatures on the performance of the catalyst, with particular interest on the saponification process. In each experiment, the reaction mixture was composed of rubber seed oil (25 mL), coconut oil 5 mL (20% volume relative to rubber seed oil), methanol (50 mL), and 3.0 g catalyst (10% mass relative to the mass of the oil). Transesterification was run at 70 oCfor 6 hours. As a control, an experiment using unsupported CaO was carried out at similar experimental conditions. After the completion of the experiment, the reaction mixture was transferred into separatory funnel for separation of biodiesel from unreacted oil. The biodiesel part was heated slowly to evaporate unused methanol, and the volume was measured. To identify the chemical components, the biodiesel was analyzed using GC-MS.
Results and Discussion
Rubber seed oil
Extraction process produced light brown rubber and highly viscous seed oil. To determine the oil content of the seed, three samples with the same mass were subjected to extraction process, and the mass of the oil obtained was weighted. From the experiments, it was found that the average oil content of the seed was 46% (w/w). This content is in agreement with the results reported by others2,16.
 |
Figure 1: Formation of soap during transesterification of rubber seed oil using CaO catalyst. Click here to View figure |
Results of Transesterification Experiment
The firstexperiment was carried out to study transesterification of rubber seed oil using CaOcalcined at 600 oC as catalyst, and very significant soap formation (saponification) was observed as shown in Figure 1. The saponification problem with the use of CaO has also been reported by others17-18. It has also been reported that leaching of Ca can be suppressed by incorporation of the CaO on solid support, such as fume silica17.
The second experiment was carried out using the CaO/SiO2 catalyst without the use of co-reactant. The experiment was conducted using the catalyst calcined at 600 oC, and it was found that the reaction yield achieved was very low (8%). The experiment using the same catalyst with the use of co-reactant, on the other hand, led to very sharp increased in the yield (83%), confirming the significant role of the co-reactant to enhance the transesterification process. The GC-chromatogram of the product is presented in Figure 2, and the list of compounds tentatively identified using the MS-library system is presented in Table 1.
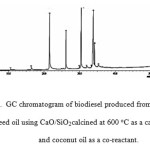 |
Figure 2: GC chromatogram of biodiesel produced from rubber seed oil using CaO/SiO2calcined at 600 oC as a catalyst and coconut oil as a co-reactant. |
Table 1: Chemical composition of biodiesel produced from rubber seed oil using CaO/SiO2calcined at 600 oC as a catalyst and coconut oil as a co-reactant
|
Peak number |
Retention time (minute) |
Molecular formula |
Compound name |
Relative percentage |
|
1 |
10.546 |
C9H18O2 | Methyl Octanoate |
0.44 |
|
2 |
16.364 |
C11H22O2 | Methyl Caprate |
1.39 |
|
3 |
21.573 |
C13H26O2 | Methyl Laurate |
21.55 |
|
4 |
26.177 |
C15H30O2 | Methyl Myristate |
10.23 |
|
5 |
29.909 |
C17H34O | 2- Heptadecanone |
0.54 |
|
6 |
30.470 |
C17H34O2 | Methyl Palmitate |
24.89 |
|
7 |
32.014 |
C18 H34O2 | Oleic Acid |
1.06 |
|
8 |
33.333 |
C20H42O | 1-Eikosanol |
0.63 |
|
9 |
33.873 |
C19 H36O2 | Methyl Oleate |
33.78 |
|
10 |
34.242 |
C19H38O2 | Methyl Stearate |
4.80 |
|
11 |
35.292 |
C18 H32O2 | Linoleic acid |
0.70 |
As can be seen in Figure 2, a total of 11 peaks are observed in the chromatogram, and 8 of them are methylesters of fatty acids composing rubber seed oil and coconut oil. The data (Table 1) also display that methyl oleateis the most prominent component of the sample, which is in accordance with the existence of oleic acid as the most abundant component of rubber seed oil as commonly reported2,19. The presence of methyl laurate suggested that lauric acid, which is the most abundant component of coconut oil20-22has been converted into biodiesel. This result also implies that coconut oil as co-reactant helped to promote the conversion of rubber seed oil into biodiesel, as has been observed for transesterification of jathropha oil7.
To investigate the effect of calcination temperatures on the performance of CaO and CaO/SiO2, both catalysts were subjected to different calcination temperatures and then tested. The results obtained are compiled in Figure 3. The results in Figure 3 display very significant role of the calcination temperatures on the catalytic activity of both CaOand CaO/SiO2. The catalysts calcined at 500 oC were found to exhibit no catalytic activity, implying that calcination at 500oC was not sufficient to activate the catalysts. The calcination temperatures of 600-800 oC resulted in sharp increase in the catalytic activity, while calcination at 900 oC was found to cause the catalysts lost their activity, most likely due to transformation of the catalysts into crystalline phase. In addition, increased calcination temperatures of CaO was found to result in reduced soap formation, although the problem was not completely solved. The results also indicate that CaO/SiO2 functioned better than unsupported CaO, and the highest conversion (92%) was achieved using CaO/SiO2calcined at 800 oC.
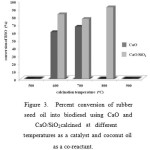 |
Figure 3: Percent conversion of rubber seed oil into biodiesel using CaO and CaO/SiO2calcined at different temperatures as a catalyst and coconut oil as a co-reactant. |
Conclusion
The results obtained demonstrate that supported CaO on rice husk silica (CaO/SiO2) is able to prevent the formation of soap during transesterification of rubber seed oil into biodiesel. The use of coconut oil as a co-reactant significantly promoted conversion of fatty acids in rubber seed oil into their corresponding methylesters. It was also found that the catalytic activity of both CaO and CaO/SiO2 was significantly influenced by calcination temperatures and at the same temperature, CaO/SiO2 exhibited higher activity than unsupported CaO. The highest yield was achieved using CaO/SiO2catalyst subjected to calcination at 800 oC
Acknowledgment
The authors gratefully acknowledge The Directorate of Higher Education, The Ministry of Research, Technology, and Higher Education, Republic of Indonesia, for financial support through research grant, Hibah Fundamental, 2016.
References
- Morshed, M., Ferdous, K., Khan, M.R., Mazumder, M., Islam, M., Uddin, M.T. Fuel. 2011, 90, 2981–2986.
CrossRef - Ramadhas, A.S., Jayaraj, S., and Muraleedha, C. Fuel 2005, 84, 335–340.
CrossRef - Marchetti, J.M., and Errazu, A.F. Biomass and Bioenergy2008, 32 (9), 892-895.
CrossRef - Prafulla, D.P. and Shuguang, D. Fuel 2009, 88, 1302–1306.
CrossRef - Leung, D.Y.C., Wu, X., Leung, M.K.H. Appl Energy 2010,87, 1083–1095.
CrossRef - Pandiangan, K.D., Jamarun, N., Arief, S., and Simanjuntak, W. Orient. J. Chem. 2016, 32, 385-390.
CrossRef - Mohadesi, M., Z. Hojabri and G. Moradi. Biofuel Res. J. 2014, 1(4), 134-138.
- Joshi, G., D.S., Rawat, B.Y., Lamba, K.I.K., Bisht, P., Kumar, N., and S. Kumar. Energy Convers. Manage. 2015,96, 258–267.
CrossRef - Sivasamy, A., K.Y. Cheah, P. Fornasiero, F. Kemausuor, S. Zinoviev and S. Miertus. ChemSusChem. 2009, 2, 278–300.
CrossRef - Ketcong, A., Meechan, W., Naree, T., Seneevong, I., Winitsorn, A., Butnark, S. and Ngamcharussrivichai, C. J. Ind. Eng. Chem. 2014, 20, 1665–1671.
CrossRef - Simanjuntak, W., S. Sembiring, K.D. Pandiangan, F. Syani, and R.T.M. Situmeang, Orient. J. Chem. 2016. 32. 2079- 2085.
CrossRef - Yap, Y.H.T., Lee, H.V., Hussein, M.Z. and Yunus, R. Biomass and Bioenergy. 2011, 35, 827-834.
CrossRef - Albuquerque, M.C.G., Urbistondo, I.J., Gonza´lez, J.S., Robles, J.M.M., Tost, R.M., Castello´n, E.R., Lo´pez, A.J., Azevedo, D.C.S., Cavalcante Jr, C.L. and Torres, P.M.. Appl. Catal., A. 2008,334: 35–43.
- Samart, C., Chaiya, C. and Reubroycharoen, P. Energy Convers. Manage. 2010,51, 1428–1431.
CrossRef - Atabani, A.E., Silitonga, A.S., Badruddin, I.A., Mahlia, T.M.I., Masjuki, H.H. and Mekhilef. S. Renewable Sustainable Energy Rev.2012, 16, 2070–2093.
CrossRef - Kouzu, M. and Hidaka, J. Fuel. 2012, 93: 1–12.
CrossRef - Endalew, A.K., Y. Kiros and R. Zanzi. Energy. 2011, 36, 2693-2700
CrossRef - Widayat, Wibowo, A.D.M. and Hadiyanto. Energy Procedia. 2013,32, 64 – 73
CrossRef - Benjapornkulaphong, S., Ngamcharussrivichai, C., and Bunyakiat, K. Chem. Eng. J., 2009, 14, 468–474.
CrossRef - Nakpong, P. and Wootthikanokkhan, S. Renewable Energy, 2010. 35, 1682–1687.
CrossRef - Pandiangan, K. D. and Simanjuntak, W. Indo. J. Chem. 2013, 13, 47 – 52.
CrossRef - Ahmad, J., Yusup, S., Bokhari, A. and Kamil, R.N.M. Energy Convers. Manage. 2014,78, 266–275.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









