Synthesis Nano Dendrimer Supramolecular with Melamine Core
Samaneh Maghsoodi, Abdoulhossien Massoudi and Mohammad Hakimi
Department of Chemistry, Payam-e Noor University of Mashhad, Mashhad, Iran.
Corresponding Author E mail: sam.maghsoodi@yahoo.com,DOI : http://dx.doi.org/10.13005/ojc/320618
Dendrimers are a family of three-dimensional polymers and in nano dimension which are characterized by spherical structure. Excellent structural properties of dendrimers have distinguished them completely from linear polymers. Dendrimers have monodispersity characteristic and their size and molecular weight is controllable exactly during synthesis such as PAMAM dendrimers.Melamine can be used for synthetic core dendrimer through various methods including divergent. Melamine and related derivatives are able to form self-assembling compound via organized intramolecular networks of hydrogen bonds and provide useful molecular scaffolding components which are exploited by the field of supramolecular chemistry; which is beyond covalent bonding. In this study, the dendrimers of generated 0.5 and 1 with ester and primary amine-terminated groups with melamine and methyl acrylate were synthesized. The Synthesized dendrimer is able to form hydrogen bonding due to its nitrogen, oxygen and hydrogen atoms which was led to supramolecule characteristic. The reaction products were identified with H NMR, and 13C NMR, FT-IR, MASS spectroscopic techniques. Also nano properties of the supramoleculs were determined by X-ray diffraction method.Supramolecular characteristics of synthesized dendrimer can be studied by shifts in H-NMR peaks and also flattening of FT-IR spectrum.The synthesized dendrimer derivatives are promising for environmental and medical applications. Also, such compounds might be reacting with transition metals as ligand and could be served as catalysts.
KEYWORDS:Dendrimer; Hydrogen bond; Supramolecule; Melamine
Download this article as:| Copy the following to cite this article: Maghsoodi S, Massoudi A, Hakimi M. Synthesis Nano Dendrimer Supramolecular with Melamine Core. Orient J Chem 2016;32(6). |
| Copy the following to cite this URL: Maghsoodi S, Massoudi A, Hakimi M. Synthesis Nano Dendrimer Supramolecular with Melamine Core. Orient J Chem 2016;32(6). Available from: http://www.orientjchem.org/?p=25561 |
Introduction
The first dendrimers, named “cascade” molecules, were produced by Vögtle et al. in 1978 [1]. Dendrimers are constructed in a stepwise manner through repeatable synthetic steps [2]. Each repetition cycle forms an additional layer of branches, called “generation” [3,4]. Two major synthetic approaches have appeared: the divergent approach in which growth starts from the inside (core) proceeding outwards, and the convergent approach proceeding “outside-in”,by first creating “dendrons” which are coupled to the core[5].Generally, synthetic chemists now have a considerable degree of control over the covalent synthesis of dendritic superstructures using a wide range of organic and inorganic methodologies[6]. Growing attention has turned to the production of dendritic structures using supramolecular synthetic methods in other words, synthesisingdendrimers held together by non-covalent (or supramolecular) interactions[7].Supramolecular chemistry lies beyond molecular chemistry based on the covalent bond and it is the chemistry of the entities generated via intermolecular noncovalent interactions[8–10]. These forces consist of hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, pi-pi interactions and electrostatic effects[11].The directionality and strength of the supramolecular bonding are significant features of systems that can be regarded as polymers and that behave based on well-established theories of polymer physics[12,13].Three main categories of supramolecular polymers can be distinguished: coordination polymers, polymers formed via π-π stacking of the monomeric units, and hydrogen bonded polymers. Hydrogen bonding interactions are very useful for the construction of supramolecular polymers due to their strength, directionality and reversibility[14].Both fully formed spherical dendrimers and individual dendritic branches (or dendrons) have been of the center of attention for their application in supramoleculardendrimer chemistry[15].Melamine and related triazine derivatives have the ability to form self-assembling, high molecular weight compound via organized intramolecular networks of hydrogen bonds and π-π aromatic ring stacking[16,17].The unique capabilities exhibited by melamine and related triazine derivatives present useful molecular scaffolding components employed by the field of supramolecular chemistry to produce a variety of sophisticated nano- or microscaled molecular compound[18,19]. In this article, a new melamine-based dendrimer is synthesized which its supramolecular and nano dimensions are studied.
Materials and Method
Materials
Melamine, methyl acrylate (Sigma- Alridch) were purified using distillation plant. Dimethyl sulfoxide (DMSO), methanol, sodium azide (Merck) were used for the synthesis.
Equipments
FT-IR analysis was performed using a SHIMADZU FTIR-8400S Spectrometer by KBr pellet technique. 1H and 13C NMR spectra were carried out on a Bruker AC 300 MHz nuclear magnetic resonance (NMR) spectrometer. The MS system employed in this study consisted of 5973 Network Mass Selective Detector of Agilent Technology (HP).
Method
Synthesis of dendrimers
Synthesis of the dendrimerG0.5 with ester terminated group
A solution of freshly distilled melamine (5.0 g) and sodium azide (0.66 g) in DMSO (50 ml) was stirred for 1h at 25°C and there was drop wised added over period of 2h to a stirred solution of methyl acrylate (10.78 ml) in DMSO (50 ml) at room temperature. The final mixture was refluxed at 70°C temperature. The solvent was removed under reduced pressure at 70°C using a rotary evaporator and the resulting cream solid washed with methanol and dried under vacuum (10-1 mm Hg, 80°C) to give the pure product (12 g, 80%) (Fig 1).
Spectral characterization of G0.5 has been determined as follows: FT-IR (υmax/Cm-1): 1651(C=O); 1H-NMR(CDCl3) δH: 6.222(3H,a), 3.60(9H,d), 3.475(6H,b), 2.515(6H,c);13C-NMR(CDCl3) δC: 172.61(d), 167.66(a), 51.80(e), 40.78(b), 39.04(c); m/z:384, 341, 294, 281,252,212,126,110,86,68.
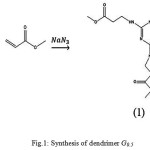 |
Figure 1: Synthesis of dendrimerG0.5 |
Preparation of amine terminated gruop G1
A solution of precursor (G0.5); (10 g) in DMSO (50 ml) was carefully added to a vigorously stirred solution of melamine (9.84 g) and sodium azide (1.32 g) in DMSO (50 ml) at 25°C. The mixture was stirred for 96h at 70°C. The solvent was removed under reduced pressure at 70°C using a rotary evaporator and the resulting cream solid washed with methanol and dried under vacuum (10-1 mm Hg, 80°C) to give a pure product (17 g, 85%) (Fig 2).
Spectral characterization of PAMAM (G1) is demonstrated as follows
FT-IR (υmax/Cm-1): 1640(C=O), 3200(NH2), 3400(NH); 1H-NMR(CDCl3) δH; 7.921(3H,d), 6.287(15H,a,e), 3.517(6H,b), 2.517(6H,c); 13C-NMR(CDCl3) δC:180.15(f), 167.64(d), 167.55( a), 158.98(e), 40.73(b), 38.99(c); m/z: 667, 650, 638, 577 ,468, 431, 381, 369, 340, 327, 296, 212,126,85.
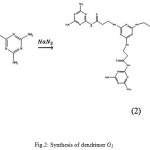 |
Figure 2: Synthesis of dendrimerG1. |
Results and Discussion
Experimental data are according to this fact that the various generation of denderimers have been synthesized.
1H and 13C NMR spectra were also fully consistent with the proposed structures. It is interesting to note that the peaks corresponding to the aromatic ring were perturbed by the presence of the dendritic branching.
H-NMR data analysis also confirmed the synthesis of dendrimers in which hydrogen of NH were appeared at 6.222 ppm and hydrogen of metoxy groups at 3.60 ppm in G0.5 while hydrogen of amino groups have been at 6.287 ppm and hydrogen of amido groups at 7.921 ppm for G1 (Fig 3).
We expected to observe peaks related to NH and NH2 groups in higher fields but they showed about 2 ppm shift toward low field which indicates hydrogen bond formation in dendrimer structure.
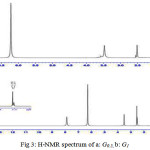 |
Figure 3: H-NMR spectrum of a: G0.5, b: G1 |
13C-NMR data analysis also confirmed the synthesis of dendrimers in which carbonyl functional groups (C = O) were appeared at 172.61 ppm andcarbone of metoxy groups at 51.80 ppmin G0.5 and carbone of amido groups have been at 167.64 ppm and carbone of aromatic ring at 158.98 , 180.15 ppm for G1 (Fig 4).
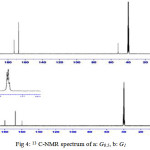 |
Figure 4: 13 C-NMR spectrum of a: G0.5, b: G1 |
FT-IR analysis for different generations indicates the presence of metoxy groups in G0.5, which is replaced by amino groups in G1 (Fig 5).
Considering frequency depletion of carbonyl group in IR spectrum it can be concluded that hydrogen formed.
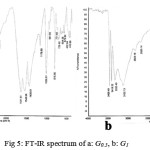 |
Figure 5: FT-IR spectrum of a: G0.5, b: G1 |
Characterisation of the novel dendritic was achieved using all standard techniques. Mass spectrometry, which is particularly important for dendritic systems, was performedusing electrospray ionisation, and the ions were observed for G0.5 and G1 no significant fragment peaks or impurities were present. The isotope distributions observed for the mass spectral ions of the larger molecules were consistent with data calculated from isotopic abundances.
According to mass spectrum (Fig 6,a) of G0.5, there are four distinct m/z peaks: at m/z 252 for losing three esteric groups from the dendrimer terminal, at m/z 212 for losing three methyl groups from the terminal of m/z 252, at m/z 126 for producing core melamine molecule and at m/z 58 for opening the melamine ring. M/z peak of G0.5 at 382 has appeared very low which shows the instability of synthesized dendrimer.
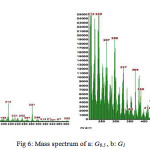 |
Figure 6: Mass spectrum of a:G0.5 ,b: G1 |
At G1 (Fig 6,b), m/z peak at 667 is similar to G0.5 and is very low which shows its instability at reaction condition and m/z peak of melamine ring opening has appeared at 468. Other distinct peaks have appeared at m/z 369, 327, 296, 267, 212, 126 and 85 which are illustrated in (Fig 7).
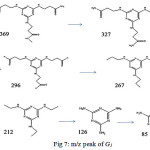 |
Figure 7: m/z peak of G1 |
According to XRD spectrum and Scherrer’s formula, synthesized dendrimer of generation 1 has nano dimension (86 nm) (Fig 8).
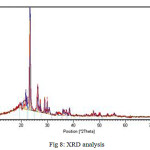 |
Figure 8: XRD analysis |
Considering the widening of IR spectrum over 3000 cm-1 and downfield shift of NMR peaks (4 ppm to over 6 ppm) for NH and NH2 it can be concluded that the synthesized dendrimer is a supramolecule.
With these observations it can be speculated that synthesized dendrimer participates in hydrogen bonding through oxygen and nitrogen atoms.
Conclusion
According to spectral evidence it can be concluded that dendrimer generation 0.5 has been synthesized through reaction of melamine as core and methyl acrylate and then it reacted with added melamine monomer and dendrimers generation 1 have been obtained with amine terminal and also the formation of supramoleculedendrimer can be assured.
Acknowledgments
We are thankful to sophisticated test and instrumentation center BuAli Research Institute and Khorasan Science and Technology Park (KSTP) of Mashhad.
References
- Buhleier,E.; Wehner,W.; Vögtle, F.Synthesis. 1978, 2, 155-158.
CrossRef - Feuerbacher,N.; Vögtle, F.Top Curr Chem.1998, 197,1-18.
CrossRef - (a) Tomalia,D.A.; Baker,H.;Dewald,J.R.; Hall,M.;Kallos,G.; Martin,S.;Roeck,J.; Ryder,J.; Smith,P.Polym J (Tokyo).1985,17, 117-132.(b) Potkin,V.I.; Shcharbin,D.;Denisov,A.A.;Paschkevich,S.G.; Bryszewska,M.;Petkevich,S.K.; Kletskov,A.V.; Lapotko,D.O.; Kazbanov,V.V.Cellular & Molecular Biology Letters.2014,19(2),243-248.
CrossRef - De Brabander-van den Berg,E.M.M.; Meijer,E.W.AngewChemInt Ed Engl.1993, 32,1308-1311.
CrossRef - Mansfield,M.L. Macromolecules. 1993,26(15),3811-3814.
CrossRef - For general reviews of supramoleculardendrimer chemistry see: (a) Narayanan,V.V.; Newkome,G.R. Top. Curr. Chem.1998,197, 19–77. (b) Smith,D.K.; Diederich,F. Top. Curr.Chem2000,210, 183–227.
CrossRef
(c) Zimmerman,S.C.; Lawless,L.J. Top. Curr. Chem.2001,217,95–120.
CrossRef
(d) Emrick,T.;Fre´chet, J.M.J. Curr. Opin. Colloid Interf. Sci. 41999,15–23. (e) Zeng,F.W.; Zimmerman,S.C. Chem. Rev.1997,97,1681–1712.
CrossRef - Lehn, J.M.VCH, Weinheim1995.
- Atwood,J.L.; Davies,J.E.D.;MacNicol,D.M.; Vögtle,F.; Lehn,J.M. Pergamon, Oxford,1996.
- Philp,D.;Stoddart,J.F.Angew. Chem. Int. Ed. Engl.1996,35,1155-1196.
CrossRef - (a) Gale,P.A.; Steed,J.W.Wiley, New York,2012. (b) Fouquey,C.; Lehn,J.M.;Levelut,A.M. Adv. Mater. 1990, 2(5), 254-257.
CrossRef - Gennady,V.O.; David,N.R.; Willem,V. Angewandte. Chemie International Edition.2007,46(14),2366–2393.
CrossRef - Sijbesma,R.P.; Meijer,E.W.Curr. Opin. Colloid Interface Sci. 1999,4, 24-32.
CrossRef - Brunsveld,L.;Folmer,B.J.B.; Meijer,E.W.; Sijbesma,R.P. Chem. Rev.2001, 101(12),4071-98.
CrossRef - Abraham,M.H. Chem. Soc. Rev.1993,22,73-83.
CrossRef - (a) Vale´rio,C.;Fillaut,J.L.; Ruiz,J.;Guittard,J.;Blais,J.C.;Astruc,D.J.Am. Chem. Soc.1997,119(10) ,2588–2589.
CrossRef
(b) Vale´rio,C.; Alonso,E.; Ruiz,J.; Blais,J.C.; Astruc,D. Angew. Chem. Int. Ed. 1999,38(12) ,1747–1751.
CrossRef
(c) James,T.D.;Shinmori,H.; Takeuchi,M.; Shinkai,S.Chem. Commun.1996, 705-706.
CrossRef
(d) Albrecht,M.; Gossage,R.A.; Spek,A.L.; van Koten,G. Chem. Commun.1998, 1003–1004.
CrossRef
(e) Boas,U.; Sontjens,S.H.M.; Jensen,K.J.; Christensen,J.B.; Meijer,E.W. ChemBioChem2002,3, 433–439.
CrossRef - (a) Seto,C.T.;Whitesides,G.M.J. Am. Chem. Soc.1990,112, 6409-6411.
CrossRef
(b) Liu,K.; Xu,Z.; Yin,M.Polymer Science.2015,46,25-54. (c) Yin,Y.; Dong,Z.;Luo,Q.; Liu,J.Polymer Science.2012,37(11), 1476-1509. - Whitesides,G.M.; Mathias,J.P.; Seto,C.T.Science. 1991,29; 254(5036),1312-1319.
- Li,X.; Chin,D.;Whitesides, G.M.J. Org. Chem.1996, 61,1779-1786.
CrossRef - Briançon et al.StudiaGeotechnicaetMechanica.2005,27 (1-2),22-32.

This work is licensed under a Creative Commons Attribution 4.0 International License.









